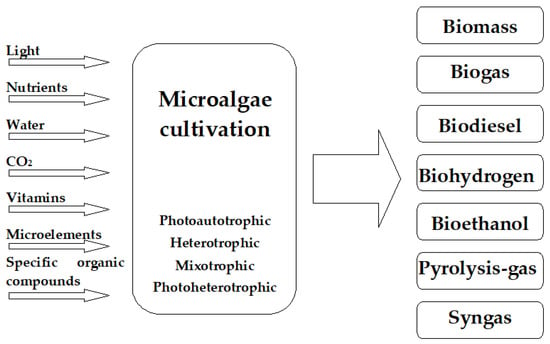
2. Microalgal Biomass as a Source of Biofuels
Microalgae can serve as a potential source of many different types of biofuels (
Figure 2). Examples include anaerobic digestion of biomass into biogas, production of biodiesel from lipids stored in algae cells and hydrogen from photobiological conversion, and lastly, gasification, pyrolysis, or direct combustion of the harvested algal biomass
[54][55][78,79].
Figure 2.
Available mechanisms for producing biofuel with microalgae.
The simplest way to use microalgae for fuel purposes involves the combustion or co-combustion of their pre-dried biomass
[56][80]. However, this solution is rarely practiced, most often in cases where the biomass of microalgae cannot be used to produce more advanced biofuels
[57][81]. Biogas and biomethane are produced during controlled, anaerobic degradation of microalgal biomass by fermentation bacteria
[58][82]. Methane fermentation is a cascade of successive biochemical transformations, including hydrolysis, acidogenesis, and methanogenesis, which are carried out by specialized consortia of microorganisms
[59][83]. In turn, biodiesel is produced via the transesterification of bio-oil extracted from microalgal biomass. This process involves the reaction of triglyceride molecules, bio-oil components, with low-molecular-weight alcohols in the presence of catalysts
[60][84]. Hydrogen production by microalgae is based on direct biophotolysis, which involves the photosynthetic production of hydrogen from water, which uses the energy of light to break down the water molecule into hydrogen and oxygen. The process is mediated by hydrogenase—a metal enzyme that catalyzes the reversible oxidation of H
2 and releases gaseous hydrogen by reducing protons
[61][85]. The basic technology for bioethanol production from microalgae entails a biochemical process in which bacteria hydrolyze the biomass and then yeast convert the sugars present in the biomass into alcohol, which is then distilled and dehydrated
[62][86]. In turn, syngas and pyrolytic gas are produced via the endothermal conversion of biomass into gas, which mainly consists of hydrogen, carbon monoxide, carbon dioxide, methane, and low-molecular-weight hydrocarbons
[63][87]. The contribution of individual products, including their qualitative composition, depends mainly on the process conditions, such as temperature, reaction time, pressure, and biomass characteristics
[64][88].
There are also reports describing technologies that stimulate and increase fatty compound storage through controlling the concentration of nitrogen compounds in the growth medium, adjusting the supply of light energy, regulating the temperature conditions, and changing the CO
2 levels
[65][66][105,106]. Essential prerequisites for cost-effective biodiesel production include the development of economically feasible technologies for separation/thickening of algal biomass, as well as oil extraction methods
[67][107]. The temperature of the extraction process is a crucial factor that directly affects the quality and quantity of the resultant oil
[68][108]. At temperatures of 60 °C and lower, higher triglyceride levels are achieved, and oil losses are reduced. Although the common practice of lipid extraction is mainly based on the use of organic solvents, some alternative and competitive technologies are still being sought. Other independent methods that aid the extraction process include mechanical, chemical, and biological treatments
[69][109]. Despite being simple, environmentally friendly, and cheap, the mechanical methods offer a low lipid recovery efficiency
[70][110]. Thus, intensive research works are in progress on the use of ionic liquids, supercritical fluids, bio-based extractants, and switchable solvents with simultaneous attention paid to reducing the energy consumption of the process by eliminating the energy-intensive drying process and the integration of multiple downstream processing steps
[71][111]. A prospective solution for lipid recovery is offered by hybrid methods, e.g., enzymatic and mechanical/solvent extraction
[72][112]. The selection of a suitable method for efficient lipid extraction largely depends on the biology and cell-wall characteristics of microalgae
[73][113].
Technologies for converting algal biomass into energy carriers can be divided into two main groups related to thermochemical and biochemical processing
[74][75][114,115]. Gasification is one of the thermochemical routes, wherein biomass is partially oxidized at temperatures ranging from 800 to 1000 °C
[76][116]. This technological solution entails reacting the biomass with oxygen and water vapor, which directly results in the generation of syngas—a mixture of CO, H
2, CO
2, N, and CH
4 [77][117]. Syngas has a low calorific value, ranging from 4.0 to 6.0 MJ·m
−3, and can be combusted directly or used as a fuel in gas turbines and gas engines
[78][118]. The properties and parameters of the microalgal biomass gasification process have been identified by several researchers. A study by Hirano et al. (1998) examined the gasification of
Spirulina sp. algae at temperatures between 850 and 1000 °C and compared the obtained energy value of syngas with that of methanol. The highest operational performance was achieved with a gasification temperature of 1000 °C
[79][119]. Minowa and Sawayama (1999) gasified
Chlorella vulgaris algae within a novel technological system, producing high-methane biofuel, as well as a fertilizer rich in ammonium nitrogen
[80][120].
A different technology for obtaining liquid biofuel is based on thermochemical liquefaction of algal biomass
[81][121]. The process is conducted at 300–350 °C and 5.0–20.0 MPa Thermochemical reactions are induced in the presence of hydrogen, which serves as a catalyst
[82][122]. The reactors are complex, both design- and technology-wise, which directly affects the construction and operation costs
[83][123]. Dote et al. (1994) successfully used the featured technology to process
Botryococcus braunii algae and obtained an oil yield of 64.0% dry matter of the algae fed into the reactor. The heating value of the bio-oil was 45.9 MJ·kg
−1, with a positive energy balance achieved across the entire process
[84][124]. In a similar experiment with
Dunaliella tertiolecta, a bio-oil recovery yield reached 42.0% dry algal biomass, and the calorific value of the resulting product was 34.9 MJ·kg
−1 [85][125].
Pyrolysis is yet another technology used to convert algal biomass into biofuel. Compared with the other methods presented in the literature, it has been widely described as a promising technology that yields very good results, inspiring high hopes for application in full-scale installations
[86][126]. Miao and Wu (2004a) used pyrolysis to extract oil from heterotrophic cultures of
Chlorella prothothecoides microalgae and achieved a bio-oil yield of 57.9% algal dry matter, with the calorific value of the resultant biofuel averaging 41.0 MJ·kg
−1 [87][127]. By comparison, Miao et al. (2004b) produced bio-oil having a calorific value of 30.0 MJ·kg
−1 at a yield of 18.0% dry
Chlorella prothothecoides biomass and 29.0 MJ·kg
−1 at a yield of 24.0% dry
Microcystis aeruginosa biomass. The algae were grown in autotrophic conditions
[88][128].
Demirbas (2006) experimented with the pyrolysis of
Chlorella prothotecoides algae, aiming to ascertain how the efficiency of the process changed with temperature. The efficiency of oil recovery from pyrolyzed algal dry matter increased from 5.7% to 55.3% as the temperature rose from 254 to 502 °C. Further increases in temperature led to a direct reduction in production yields. The heating value of the harvested bio-oil peaked at 39.7 MJ·kg
−1 [89][136]. Many of the findings published in the literature seem to indicate that bio-oil extracted from algal biomass is higher in quality than the biofuel obtained through pyrolysis of lignocellulosic plants
[89][90][136,137].
Algae can also serve as a source of ethyl alcohol. It has been demonstrated that
Chlorella sp. algae are viable candidates for effective alcoholic fermentation due to their high starch content (approximately 37.0% dry matter). Experimental data indicate a carbohydrate-to-ethanol conversion rate of 65.0%
[91][138]. Ueno et al. (1998) corroborated the feasibility of ethanol production using microalgae harvested from a heterotrophic culture. The productivity of the alcoholic fermentation process performed at 30 °C was 450 μmol·g
−1 dry matter
[92][139]. The research carried out to date confirms that the production of ethyl alcohol from algal biomass can be technologically and commercially viable under specific conditions. In most cases, however, alcoholic fermentation is used as a supplemental technological step for processing algal biomass residues from the oil extraction process
[93][140].
Hydrogen is a naturally occurring molecule that can serve as a clean and efficient energy carrier. Studies have confirmed that microalgae possess the genetic, metabolic, and enzymatic properties required to produce H
2 through biochemical conversion
[94][149]. Under anaerobic conditions, eukaryotic algae generate hydrogen as an electron donor in their metabolic pathways as part of the CO
2 fixation process. This mechanism has been found to occur both in the light and in the absence of any light sources
[95][150]. During photosynthesis, algae convert the water molecule into a hydrogen ion (H
+) and oxygen. The H
+ ions are then converted by hydrogenase into molecular hydrogen (H
2) under anaerobic conditions
[96][151]. It has been demonstrated that, if photosynthesis is initiated and oxygen is present in the photosynthetic environment, inhibition of the key enzyme (hydrogenase) follows shortly, directly affecting hydrogen production by algae
[97][152].
Most of the scientific publications on this subject reported that the single-cell
Chlamydomonas reinhardtii algae, commonly found in soil and saltwater, can produce H
2 with high efficiency
[98][99][153,154]. The hydrogen production capacity of 21 green algae species in an isolated anaerobic environment was also examined. The most productive strains were
C. reinhardtii,
C. euryale, C. noctigama, C. vectensis, C. pyrenoidosa, Oocystis, D. subspicatus, and
P. subcapitata. Publications reported H
2 yields of 90–110 cm
3 H
2·dm
−3 for these organisms, with even higher levels of 80–140 cm
3 H
2·dm
−3 reached in some cases
[100][155]. Ample publications have shown that
Platymonas subcordiformis algae can be used for the technological production of biohydrogen. The method employs alternating dark and light cycles with external carbon dosing, and it can produce H
2 yields of 78.0 cm
3 H
2·dm
−3 to as high as 126 cm
3 H
2·dm
−3 [101][102][156,157].
Methane fermentation can also be employed to convert algal biomass into a gaseous energy carrier through biochemical processes. According to available estimates, the conversion of algal biomass into biogas is a highly cost-effective and commercially viable technological solution comparable to cellular lipid extraction in terms of harvested energy
[103][104][164,165]. In addition to high-energy biogas, the process also produces digestate, which can be used directly as a fertilizer for terrestrial plants or reintroduced into the algal biomass route as a medium component after simple processing
[105][166].
The practical limitations of technological processes involving methane fermentation of algae may stem from their biochemical composition. Algal biomass mostly consists of proteins and, thus, may lead to deficient C:N ratios. This problem can be greatly alleviated through the co-digestion of the algal biomass with organic substrates rich in carbon compounds. Yen and Brune (2007) achieved a substantial increase in methane production by co-digesting cellulose waste with algal biomass. The methane production rate rose to 1170 ± 75 cm
3·dm
−3·day
−1 at a 1:1 ratio of organic waste and algal biomass, as compared to 573 ± 28 cm
3·dm
−3·day
−1 achieved for mono-digestion of algae alone
[106][167].
The high protein content of the algal biomass may lead to an increased production of free ammonia, which is toxic to the methane-fermenting microorganisms. Methanogenesis can also be inhibited by the sodium ions present in the algal biomass from saltwater-based cultivation systems. However, some studies show that anaerobic sludge microorganisms can be adapted and incorporated into the process for the efficient digestion of marine algal biomass
[107][108][168,169].
Many researchers have argued that methane fermentation is the most promising and effective method for producing energy from algae. Sialve et al. (2009) found that, given suitable operating conditions, methane fermentation as a primary method of algal biomass processing is more economical than systems that incorporate lipid extraction and anaerobic processing of post-extraction residues
[104][165]. Other findings suggest that the balance of methane fermentation unit operations is the most effective in terms of both the economy of the process and the pollution levels
[109][180]. Studies have indicated that methane fermentation may be the most practical means of converting algal biomass into energy. However, Börjesson and Berglund (2006) noted that energy inputs and environmental impact varied greatly between the different methane fermentation technologies
[110][181]. As such, an environmental life-cycle assessment (LCA) is necessary for a complete and objective evaluation of each process
[111][182].
To meet the current challenges related to the circular bioeconomy, it is necessary to change the approach to biorefinery processes
[112][183]. Technological, economic, and environmental efficiency improvements can be achieved by simultaneously producing many high-value products other than biofuels
[113][114][184,185]. Research and development works must, therefore, be focused on finding new, more complex, and integrated production processes. Although various strategies have been proposed for converting algal biomass into fuel and fine chemicals, none have been proven to be economically viable and energy balanced
[115][186]. Therefore, other, valuable biological products should also be searched for. In this context, the concept of microalgae biorefineries emerged with the concept of recovering multiple products from one operating process. Considering the biorefinery complexity index (BCI) as an indicator of technical and economic risk, one of the most promising seems to be the biorefinery platform based on microalgal biomass conversion into fuels, food, dietary and feed supplements, fertilizers, and pharmaceuticals
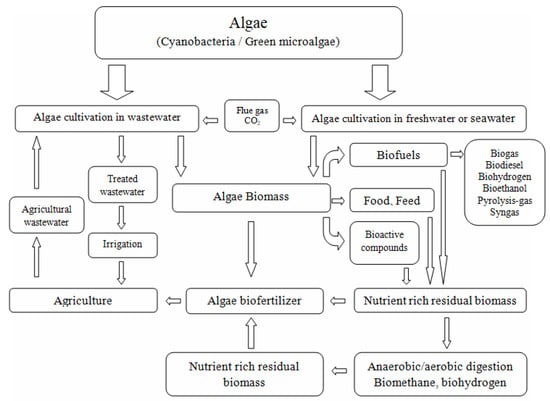
[116][187]. A schematic diagram of a comprehensive biorefinery approach to the processing of microalgal biomass is presented below (
Figure 3).
Figure 3.
A schematic diagram of a comprehensive biorefinery approach to microalgal biomass processing.
3. Systems of Microalgae Species Cultivation for Biofuel
The growth rate of microalgae and their composition is influenced by the growth conditions and the species employed
[117][118][119][188,189,190]. Many classification schemes categorize methods and technologies used to cultivate algae for biofuel
[120][121][191,192]. Due to the specific nature of microalgae, the most important scheme divides the systems on the basis of the nutrient source and the type of biochemical processes used to grow the algal biomass rapidly. With this criterion in mind, cultures can be divided into four main types: photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic
[122][193].
In a photoautotrophic culture, microalgae use light as their source of energy, as well as carbon dioxide and water to synthesize organic compounds
[123][198]. This type of algae culture is most commonly used for commercial applications
[124][199]. Studies have shown photoautotrophic cultures to exhibit great variability in algal biomass lipid content, with values ranging from 5% to 68% depending on the tested strain. A study with
Chaetoceros calcitrans CS 178 showed a lipid production rate of r
LIP = 17.6 mg·dm
−3·day
−1 and a final lipid content of 39.8% dry matter
[125][91]. In contrast, a
Botryococcus braunii UTEX 572 culture ended in the lipid production yield of r
LIP = 5.5 mg·dm
−3·day
−1 [124][199]. The highest productivity was obtained in a study that tested the impact of high concentrations of CO
2 on biomass growth and lipid synthesis in
Chlorella sp. culture. The final lipid concentration reached 32–34% cell dry matter, with a maximum lipid production rate of r
LIP = 179.8 mg·dm
−3·day
−1 [126][200].
Like bacteria and fungi, some microalgae species are capable of heterotrophic growth using organic carbon sources, such as glucose and glycerol
[127][128][196,201]. Heterotrophic cultivation can be used to avoid the problem endemic to photoautotrophic systems, i.e., overgrown photobioreactor surfaces and the microalgal growth blocking its own light source, thus limiting the energy supply necessary for efficient photosynthesis, biomass growth, and lipid synthesis
[123][198]. Heterotrophic cultures are characterized by higher growth rates and final biomass/lipid concentrations than the phototrophic or mixotrophic cultures. For example, a heterotrophic culture of
Crypthecodinium cohnii—a strain known for its ability to biosynthesize omega-3 acids—grown on a complex medium of glucose, acetic acid, and yeast extract, produced final concentrations of 109 g·dm
−3 dry biomass and 61 g·dm
−3 lipids in the culture
[129][202].
Changing the culture conditions from photoautotrophic to heterotrophic can increases lipid content per cell dry matter for some microalgal strains. For example, a 40% increase in lipid content was observed in a
Chlorella protothecoides culture after the cultivation scheme was changed from photoautotrophic to heterotrophic
[130][96]. In another study, changing the conditions from phototrophic to heterotrophic led to an over tenfold reduction in the final biomass concentration in a
C. vulgaris ESP-31 culture
[131][203]. In the lipid analysis of
Chlorella protothecoides cultures, Caporgno et al. (2019) achieved fatty acid contents at 11.8% ± 0.1% dry weight (DW) and below 6% DW under heterotrophic and photoautotrophic conditions, respectively
[132][204]. Sim et al. (2019) also observed an increased lipid production by
Chlorella protothecoides. It reached 18.4% ± 0.4% DW under conditions of the heterotrophic culture and 15.1% ± 0.3% DW under photoautotrophic conditions
[133][205]. Shen et al. (2019) demonstrated an increase in fatty acid production by
Chlorella vulgaris that ranged from 14.9 ± 2.1 mg·dm
−3·day
−1 under photoautotrophic conditions to 51.4 ± 14.6 mg·dm
−3·day
−1 in the heterotrophic culture
[134][206]. Li et al. (2016) obtained maximum biomass production in the photoautotrophic culture of
Chlorella sorokiniana, reaching 0.36 ± 0.01 g·dm
−3 at a specific growth rate of 0.60 ± 0.01 day
−1. Under heterotrophic conditions, the respective values were 2.78 ± 0.06 g·dm
−3 and 1.56 ± 0.02 day
−1 [135][207]. In turn, Zheng et al. (2012) proved that the growth rate, cell density, and productivity of heterotrophic
Chlorella sorokiniana were 3.0, 3.3, and 7.4 times higher than their phototrophic counterpart, respectively
[136][208]. Lastly, Li et al. (2014) achieved the lipid content at 9.0% DW in the photoautotrophic culture of
Chlorella sorokiniana and at 6.2% to 17.6% DW in the heterotrophic cultures
[137][209].
Microalgae have been shown to take up many different organic carbon sources, including glucose, acetate, glycerol, fructose, sucrose, lactose, galactose, and mannose
[138][139][97,210]. De Swaaf (2003) presented a study examining the use of different organic substrates in a heterotrophic culture, utilizing acetic acid and its feeding regime in a pH-controlled culture to grow
Crypthecodinium cohnii [129][202]. This technological solution resulted in very high values of the final productivity parameters, i.e., final cell dry matter concentration at 109 g·dm
−3 and 61 g·dm
−3 lipids in the culture. Other studies showed
Chlorella protothecoides to be capable of growth in a batch culture with crude glycerol as the sole carbon source in the medium, with the final biomass concentration at 23.5 g·dm
−3 and the final lipid concentration at 14.6 g·dm
−3 after a 6 day cultivation
[140][211]. In turn, a semi-continuous batch-fed regime allowed increasing the lipid production rate to 3 g·dm
−3·day
−1 [140][211].
However, heterotrophic cultivation certainly has its disadvantages, including the frequent contamination of the culture with other strains of microalgae, fungi, and bacteria, reducing the final productivity of the technology and, in some cases, inhibiting fermentation
[130][141][142][96,212,213]. One instance of this problem was described by Zhang et al. (2012) who investigated the impact of bacterial contamination on the dry biomass yield and lipid productivity in a heterotrophic culture of
Chlorella pyrenoidosa, with soybean-processing wastewater used as a medium. On the one hand, the introduction of bacteria improved nitrogen and phosphorus degradation rates while reducing the chemical oxygen demand. On the other hand, the bacteria also reduced the final concentrations of microalgal biomass and lipids
[143][214]. One of the methods used to avoid contamination of heterotrophic microalgal cultures entails spiking the medium with antibiotics, such as chloramphenicol
[144][215].
In the mixotrophic cultivation, microalgal cells perform photosynthesis with simultaneous uptake of organic and inorganic carbon substrates
[145][216]. Microalgae absorb organic compounds, and the CO
2 released through respiration is captured and reused as a substrate for photosynthesis
[146][217]. Unlike phototrophic and heterotrophic systems, the mixotrophic cultivation is rarely employed for the production of microalgae-derived bio-oil. One example of a mixotrophic culture was found in a study by Bhatnagar et al. (2011), who examined the growth rates of
Chlamydomonas globosa, Chlorella minutissima, and
Scenedesmus bijuga in the three most common cultivation modes. Supplementing
Chlamydomonas globosa, Chlorella minutissima, and
Scenedesmus bijuga cultures with 1% (
w/v) glucose was found to increase mixotrophic biomass yields 9.4, 6.7, and 5.8 times (respectively) compared to the phototrophic culture and 3.0, 2.0, and 4.4 times compared to the heterotrophic culture
[147][218]. Yu et al. (2009) obtained similar results, demonstrating that the growth rates of
Nostoc flagelliforme biomass in glucose-amended media were the highest in the mixotrophic culture, with productivity values 5.0 and 2.3 times those obtained in the phototrophic and heterotrophic cultures, respectively
[148][219].
Though microalgal oil yields are in large part determined by the choice of strain, the heterotrophic cultivation is the most effective solution in terms of the final operational performance, i.e., the biomass concentration in the system and lipid content in cells. As such, the heterotrophic method has generated strong interest among companies involved in the commercialization of bioenergy technologies and research teams working to develop such systems
[149][220]. The most serious drawback of this scheme is the risk of culture contamination with other microorganisms, including other microalgae, which leads to severe complications with the operation of industrial-scale installations
[144][215]. Moreover, the high cost of pure organic carbon sources limits the utility of this cultivation mode to the production of secondary or primary metabolites with a high market value
[150][221].
Photoautotrophic cultures are the most widespread mode of cultivation, easy to scale up through the use of open or hybrid systems
[151][222]. It is also a promising method, due to the capability of photoautotrophic microalgae for the uptake of waste CO
2, such as that generated by cogeneration plants, breweries, or biogas plants. However, the oil yields produced via this method are usually vastly inferior to the heterotrophic cultivation, with slow cell growth and low biomass productivity as the main reasons. Nevertheless, with this mode being cheaper to scale up, it is highly attractive to investors despite the flaws.
The defining feature of photoheterotrophic cultivation is the use of light, required for the absorption and decomposition of organic carbon. The main difference between mixotrophic and photoheterotrophic modes is that the latter requires light as an energy source, whereas mixotrophic cultivation uses organic compounds for the same purpose. Therefore, photoheterotrophic cultivation requires a combined supply of carbohydrates and light
[127][196]. Although photoheterotrophic systems can be used to increase the production of certain expensive secondary metabolites, the method has not found use in the production of biodiesel, as is the case with mixotrophic microalgal cultures
[152][197].
Prior to undertaking any metabolic engineering work in microalgae, it is necessary to understand the key enzymes involved in the metabolic pathway and the rate-limiting enzymes. Many advances have been made toward understanding lipid metabolism and regulatory factors in soybean and rapeseed, but the lipid production in microalgae at a molecular level is currently very poorly understood. The first step in de novo synthesis of triacylglycerol in microalgae starts in the plastid, where pyruvate is produced from glycolysis and the Calvin cycle. The pyruvate is converted into acetyl-CoA by the pyruvate dehydrogenase complex (PDC). Acetyl-CoA is converted into malonyl-CoA by acetyl-CoA carboxylase (ACCase). Acetyl-CoA carboxylase is the rate limiting enzyme for lipid biosynthesis
[153][223]. Malonyl-CoA is converted into malonyl-ACP by malonyl-CoA transacylase (MAT)
[154][224]. Malonyl-ACP and acyl-ACP are converted into 3-ketoacyl-ACP by 3-ketoacyl-ACP reductase (KAS) in the fatty acid synthesis cycle. 3-Ketoacyl-ACP is converted into 3-hydroxyacyl-ACP by 3-ketoacyl-ACP reductase (KAR). 3-Hydroxyacyl-ACP is converted into
trans-enoyl-ACP by 3-hydroxyacyl-ACP dehydratase (HD).
trans-Enoyl-ACP is converted into acyl-ACP by enoyl-ACP reductase (ENR). Acyl-ACP is converted into free fatty acids (FFAs) by fatty acyl-ACP thioesterase (FAT)
[155][156][225,226]. The FFAs are transferred into the cytosol and then endoplasmic reticulum for conversion into triacylglycerol (TAG) in the microalgae. The free fatty acids are converted into acyl-CoA by long-chain acyl-CoA synthetase. Acyl-CoA and glycerol 3-phosphate are converted into lysophosphatidic acid by glycerol 3-phosphate acyltransferase (GPAT). Lysophosphatidic acid is converted to phosphatidic acid by lysophosphatidic acid acyltransferase (LPAT). Phosphatidic acid is converted into diacylglycerol by phosphatidic acid phosphatase (PAP). Diacylglycerol is converted into triacylglycerol (TAG) by diacylglycerol acyltransferase (DGAT). Triacylglycerol forms the TAG lipid body
[156][157][226,227].
Hydrogen production in biological processes conducted by algae is based on the direct biophotolysis, which consists of the photosynthetic production of hydrogen from water, in which the energy of light is used to break the water molecule into hydrogen and oxygen
[158][228]. It takes place mainly due to hydrogenase, which catalyzes the reversible oxidation of H
2 and releases gaseous hydrogen by reducing protons
[159][160][229,230]. Two transmembrane peptide complexes are responsible for hydrogen production in the photolysis process by microalgae: photosystem I (PSI) and photosystem II (PSII). The exposure of both complexes to solar radiation results in a water molecule breakdown. Then, O
2 is produced by PSII, while PSI uses the electrons generated in this process to reduce CO
2 and build cellular material (aerobic conditions), or the electrons are transferred by ferredoxin to hydrogenase and used for hydrogen production
[161][162][231,232]. Another biochemical process led by algae to produce hydrogen is indirect biophotolysis. It has been proven to occur in the organisms of cyanobacteria, which accumulate carbohydrates resulting from CO
2 reduction as a result of photosynthesis, which in turn are decomposed by fermentation mediated by photosystem I. The PSI proteins transfer electrons to ferredoxin using light energy
[158][162][228,232]. In the indirect biophotolysis process, an important role is played by carbon dioxide, which is a carrier of electrons and protons formed during the water molecule degradation, and by enzymes, including two NiFe hydrogenases and nitrogenase, which catalyze atmospheric nitrogen reduction to ammonia with simultaneous proton reduction and hydrogen release
[163][164][233,234].