Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Jamir Pitton Rissardo.
Asterixis is a subtype of negative myoclonus characterized by brief, arrhythmic lapses of sustained posture due to involuntary pauses in muscle contraction. Asterixis is not specific to any pathophysiological process, but it is more commonly reported in hepatic encephalopathy, renal and respiratory failure, cerebrovascular diseases, as well as associated with drugs that could potentially lead to hyperammonemia, such as valproic acid, carbamazepine, and phenytoin.
- asterixis
- anisosterixis
- mini-asterixis
- myoclonus
- hyperkinetic
- encephalopathy
- drug induced
- movement disorder
1. Introduction
Asterixis is a disorder of motor control, defined as sudden, brief, arrhythmic lapses of sustained posture due to involuntary interruption in muscle contraction [1,2][1][2]. This condition involves a specific form of negative myoclonus, characterized by momentary loss of muscle tone in agonist muscles, followed by a compensatory jerk involving antagonistic muscles [3]. Asterixis was originally described as a “liver flap” in the 1940s by liver specialists at the Thorndike Laboratory at Boston City Hospital, who noted the movement disorder in their patients [4]. It was also observed that this abnormal movement was common in metabolic encephalopathies and not only in patients with hepatic disease [5]. Reportedly, with the help of Father Cadigan, a Jesuit classic scholar from Boston College, Adams and Foley initially created a term to explain the asynchronous flapping: “anisosterixis”, from the ancient Greek, where an = negative, iso = equal, sterixis = solidity or firmness. Foley realized the word was too complicated to be used in clinical practice and simplified it to “asterixis” [4,5][4][5].
Asterixis is acknowledged as a significant albeit non-specific neurological manifestation linked with numerous conditions, with metabolic encephalopathies being the most prevalent culprit. It also has been reported in renal insufficiency and respiratory failure, both with hypercapnia and hypoxia, structural brain lesions due to cerebrovascular accidents, tumors, subdural hematoma, epidural abscess, polycythemia, septic encephalopathy, hyperviscosity, and medications [6,7][6][7].
It is possible to elicit asterixis against gravity, but when the wrist is in dorsiflexion, gravity accentuates the downward movement of the hand. There are early reports from Adams and Foley dating from 1953 describing asterixis also in proximal muscles of the upper limbs, lower limbs, neck, face, and tongue. It is also known that depending on the cause, asterixis could be fully reversible. Generally, when toxic-metabolic encephalopathy is treated and resolved, for example, so does the asterixis [7].
2. Etiology
Unilateral or bilateral asterixis can manifest, often with asynchronous, irregular, and varying frequency and amplitude. The clinical history of the possible underlying illness or toxic/metabolic process should be a starting point for further investigations, along with suggestive clinical signs, considering that asterixis is a highly non-specific sign associated with multiple causes. In 1973, Young and Shahani were the first to describe unilateral asterixis, and they classified involuntary movements as a form of “mini-asterixis” and the pauses as negative myoclonus [8]. Unilateral asterixis could arise from focal lesions in the thalamus, although cases of lesions in the midbrain, parietal cortex, and frontal cortex leading to unilateral asterixis have been reported [9]. In rare occasions, bilateral asterixis could be secondary to a unilateral lesion, for example, subdural hematoma causing mass effect [2]. A study of 45 cases with asterixis revealed ischemic and hemorrhagic disorders of the central nervous system (CNS) to be the most frequent causes of asterixis (95.5%), and the thalamus was the most frequent localization for unilateral asterixis (54%) [10]. Bilateral asterixis is commonly linked to metabolic encephalopathies, particularly of hepatic origin. Patients with cardiac and respiratory failure, uremia, electrolyte imbalances (primarily hypoglycemia, hypokalemia, and hypomagnesemia), and drug intoxication may also exhibit bilateral asterixis. An array of drugs can induce asterixis, with phenytoin intoxication being the most frequently reported, followed by benzodiazepines, barbiturates, valproate, gabapentin, carbamazepine, lithium, ceftazidime, and metoclopramide [11]. In terms of other generalized encephalopathies, asterixis has also been associated with cerebral malaria, probably due to impaired microvascular circulation [12], Creutzfeldt–Jacob disease, where it could be secondary to non-inflammatory changes associated with malformed proteins and encephalitides to brain inflammation caused by infection [13] and viral encephalitis probably in the context of neuroinflammation [14]. Cerebral trypanosomiasis has also been associated with bilateral asterixis in a case report. In the latter, treatment with eflornithine led to the resolution of obtundation and asterixis and considerable resolution of brain magnetic resonance imaging (MRI) abnormalities. In this way, the early consideration of non-CNS sources of infection in patients initially presenting with encephalopathy and asterixis is important. Epidemiological factors such as travel history, occupation, sexual contacts, and vaccination history should be taken into account to guide microbiological investigations [7]. Asterixis is more often noticed in the upper extremities than in the lower. Nevertheless, there have been reports of asterixis that are more noticeable on the lower extremities than the upper. This particular presentation could be explained by altered mental status and the patients’ difficulties in obeying commands and performing dorsiflexion of the wrists. However, the predominance of asterixis in lower extremities has also been reported in alert patients. It is also commonly seen that as asterixis worsens in the upper extremities, it gradually becomes more prominent in the lower extremities [7].3. Pathophysiology
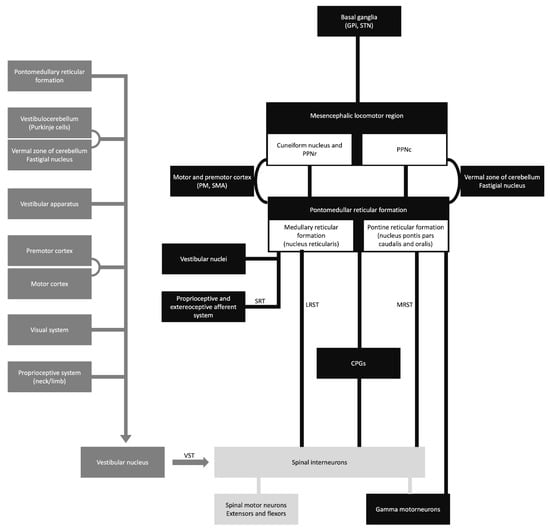
The vestibulospinal, reticulospinal, and rubrospinal tracts are the main tracts related to the postural stability or tonic control of the extremities. The modulation of these tract functions is controlled by supratentorial structures (Figure 1) [15]. In this context, the cerebello-rubral and vestibulocerebellar fibers converge to the ventrolateral nucleus of the thalamus and some to the prefrontal cortical area [15]. Another interesting fact is that some muscle tone and postural regulation occur by the medial frontal cortex, which projects to the brainstem reticular formation [15]. The transient motor symptoms and the common bilateral presentation suggest that the postural tone control is mainly bilateral.
Figure 1. Schematic diagram of the sensory-motor control. CPGs, central pattern generators; GPi, globus pallidus internus; LRST, lateral (medullary) reticulospinal tract; MRST, medial (pontine) reticulospinal tract; PM, premotor cortex; PPNc, caudal region of pedunculopontine nucleus; PPNr, rostral region of pedunculopontine nucleus; SMA, supplementary motor area; SRT, spinoreticular tract; STN, subthalamic nucleus; and VST, vestibulospinal tract.
Asterixis, initially observed by Adams and Foley in 1949, is usually a bilateral tremor characterized by flapping movements. It is often considered an indicator of subcortical negative myoclonus, a rhythmic motor phenomenon [16]. The presence of this condition has been reported in various metabolic encephalopathies as an adverse effect of drugs and structural abnormalities. Unilateral asterixis has been reported in structural brain lesions [10].
The pathophysiology of asterixis is still unknown. It was hypothesized as a disturbance of the ascending activating systems, affected by conditions like encephalopathy and related to lesions in the thalamus and midbrain [17]. Possible brain areas that can explain the pathophysiology of asterixis are the parietal lobe and midbrain. These cortical regions are mainly related to sensorimotor integration, and their dysfunction can lead to the receptive inattentiveness of incoming information [17].
4. Diagnosis
4.1. Clinical Assessment
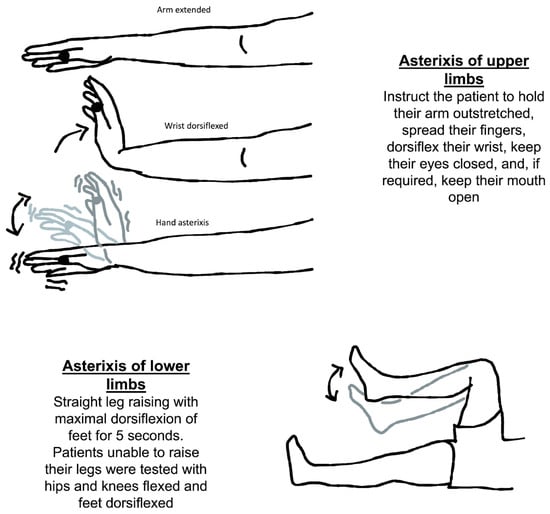
The usual method for eliciting asterixis is to instruct the patient to hold their arm outstretched, spread their fingers, dorsiflex their wrist, keep their eyes closed, and, if required, keep their mouth open (Figure 2). Then, looking for any abnormally brief downward flaps of the hands that return quickly to their previous posture is possible. If it is not obvious immediately, the patient can be instructed to keep their arms straight while the examiner softly extends their wrist in a sweeping motion. There is a significant latent period between adopting the posture and the beginning of the asterixis, so it is important to wait at least thirty seconds before concluding that the sign is absent.
Figure 2.
Neurological assessment of asterixis of the upper and lower limbs.
Another way to check for asterixis is to have the patient lie on their back on the bed with both their knees bent for evaluation of symmetry. The patient should be told to let their legs down. When the legs fall to the sides, the feet should be flat on the table. It is important to observe any flapping of the legs at the hip joint. The knees repeatedly come back together as a result of this. It has also been discovered that asterixis cannot be properly induced without evaluating the limbs against gravity [20][18].
4.2. Asterixis in Any Skeletal Muscle
Adams and Foley described that asterixis can occur in any skeletal muscle in which voluntary musculature is required to maintain posture [21][19]. The most common location of asterixis is the wrist, but severe cases can reveal the presence of this phenomenology in the tongue, lips, and eyelids [7]. Interestingly, asterixis usually occurs asynchronously on either side of the body, except when it involves the facial muscles [21][19].
One location of asterixis that has still not been reported is the extraocular muscles. The clinical detail of the movement and its amplitude were probably some factors associated with the scarce description in the literature. Another hypothesis could be that asterixis simply does not occur in extraocular muscles.
4.3. Diagnostic Assessment
Complete blood cell count, electrolytes, glucose, renal function tests, liver function tests, and arterial blood gas analysis are the recommended laboratory studies to rule out metabolic conditions associated with asterixis. In individuals using medications, if a patient’s history points to drug intoxication, it is important to investigate drug-induced asterixis. Neuroimaging can help locate possible lesions in the CNS associated with asterixis. The mean frequency of movements has been historically reported to be around 3–5 Hz. However, in clinical practice, it is usually less, around the range of 0.5 to 2 Hz. Very fine asterixis affecting the fingers can be easily confounded with tremors [2]. The main differences between asterixis and tremor will reside in the phenomenology of the movement. Asterixis is usually antigravitational, but, in some cases, the differentiation can only be appreciated with electrodiagnostic studies. EMG features include a 35 to 200-millisecond electrical activity pause in many muscles. For example, the affected limb may jolt back into place or be impacted by gravitational or tendinous elastic stresses. This is followed by sudden motor unit activation [3]. In this context, the silent period locked averaging method was described by Ugawa et al. and uses a backward averaging methodology to analyze asterixis [23][20]. This approach can be used to investigate the causes of asterixis as well as the many types of EMG silences that accompany it.5. Differential Diagnosis
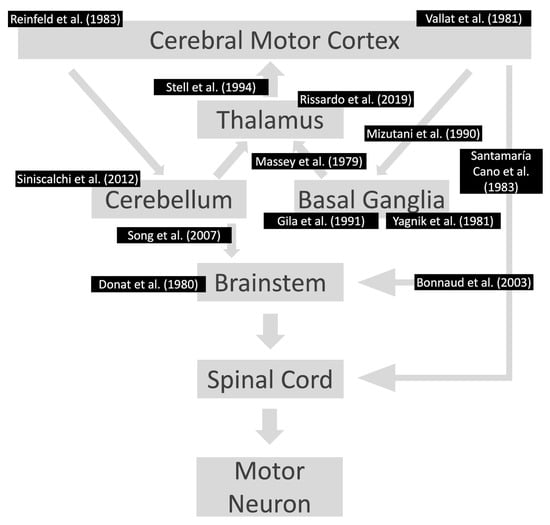
Asterixis has been extensively studied in adults, with an estimated incidence varying from 0.47 to 8.75% of neurological consultations [7,27][7][21]. The differences between the percentages can be explained by two factors. First, patients usually do not spontaneously report asterixis. Second, some of the studies performed detailed examinations of all the patients where the investigator actively searched for asterixis in the physical exam. Interestingly, the literature regarding asterixis in the pediatric population is scarce. Aravamuthan et al. found only 0.06% of the pediatric individuals presented with asterixis in a tertiary care pediatric hospital [28][22]. All the cases of pediatric asterixis were side effects of medications, and the neurologists were consulted for complaints unrelated to asterixis, such as requested a second opinion regarding non-improvement in seizure attacks after optimal therapy [28][22]. The presence of bilateral asterixis at presentation suggests metabolic abnormalities and side effects of medications. The metabolic conditions associated include but are not limited to hepatic encephalopathy, renal failure/azotemia, respiratory failure, electrolyte disturbance, heart failure, Wilson’s disease, and hypoglycemia. In the cases of drug-induced asterixis, there are reports of individuals using alcohol, barbiturates, carbamazepine, antipsychotics (clozapine, lithium), phenytoin, gabapentin, metoclopramide, and levodopa. In cases of unilateral presentation of negative myoclonus, focal structural brain lesions in the genu and the anterior portion of the internal capsule or ventrolateral thalamus should be investigated (Figure 3) [7,20,31,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53][7][18][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38]. In this context, cerebellar lesions sometimes cause ipsilateral asterixis, which can be explained by the decussation of dentato-rubro-thalamo-cortical fibers before they pass through or create synapses with the red nucleus. In this way, it is imperative to conduct a complete neurological examination in these patients because accurate diagnosis frequently relies on careful attention to neurological signs and symptoms. There is a broad differential diagnosis for cerebellar pathologies, and alterations in coordination, eye movements, and balance could indicate an underlying vascular lesion for example which should be promptly managed [54][39]. No unilateral asterixis reports associated with contralateral brain lesions have been reported, with good anatomy documentation (imaging or autopsy) and case-by-case documentation of normal blood chemistry.
Figure 3. Lesions already reported in the motor pathway associated with asterixis. The references are according to the area affected in the motor pathway. Notably, the individuals had contralateral motor symptoms at the location of the lesions. References: [20,31,39,41,42,43,44,45,46,48,50,51,53][18][23][24][26][27][28][29][30][31][33][35][36][38].
One crucial differential diagnosis of asterixis is pseudoasterixis. Subtle movements may trigger pseudo-asterixis so that it can simulate asterixis. Pseudoasterixis is defined as brief, rapid, voluntary action tremors of the hands and fingers, elicited by slow flexion and extension movements of the hands at the wrists while keeping the fingers in full hyperextension. Another fact to differentiate asterixis from pseudo-asterixis is that the patient is aware of the hand twitching when tested in pseudoasterixis [55][40].
6. Management
6.1. Asterixis in Hepatic Encephalopathy
Hepatic encephalopathy is defined as a spectrum of neuropsychiatric abnormalities in patients with liver dysfunction after the exclusion of brain disease [133][41]. A raised serum ammonia level is the classic laboratory abnormality reported in patients with hepatic encephalopathy. Triphasic and high-amplitude low-frequency waves are classic EEG changes associated with hepatic encephalopathy. Neuroimaging should be performed to rule out intracranial lesions when the diagnosis of hepatic encephalopathy is in question [134][42].
Because of the increased dietary fiber content, a natural cathartic, and decreased levels of aromatic amino acids, diets containing vegetable proteins appear to be better tolerated than diets rich in animal proteins, especially proteins derived from red meats. Aromatic amino acids are precursors of the false neurotransmitters, tyramine and octopamine, which are thought to inhibit dopaminergic neurotransmission and worsen hepatic encephalopathy. Lactulose appears to inhibit intestinal ammonia production by several mechanisms. It produces acidification of the gut lumen by conversion of lactulose to lactic acid and acetic acid [135][43]. This enhances the conversion of ammonia (NH3) to ammonium (NH4+); owing to the resultant relative impermeability of the membrane, the NH4+ ions are not easily absorbed and hence get trapped in the colonic lumen and reduce the plasma NH3. Gut acidification inhibits the ammoniagenic coliform bacteria, which leads to increased levels of non-ammoniagenic lactobacilli. Patients should take sufficient lactulose to have two to four loose stools daily.
6.2. Uremic Encephalopathy
Patients with uremic encephalopathy may present with stimulus-sensitive myoclonus, asterixis, or both, which generally improve after dialysis or renal transplantation [140][44]. Most patients with chronic renal disease will not have complaints, but their detailed neurological examination will show asterixis. Also, the chronically elevated creatinine and urea levels in individuals undergoing dialysis are probably associated with the development of asterixis, and sometimes, even with an optimal dialytic regimen, asterixis will not improve.6.3. Genetic Etiologies and Deep Brain Stimulation
Miyata et al. reported an individual presenting with asterixis and dystonia with a KMT2B mutation. His neurological symptoms were severe, and deep brain stimulation of the globus pallidus was performed, showing a significant improvement in dystonia and asterixis. Improving both movement disorders can suggest a common pathologic mechanism for these two pathologic movements [141][45]. The globus pallidus stimulation and significant asterixis improvement suggest that the postural stability and tonic control may be partially modulated by the globus pallidus [142][46].6.4. Others
In cases of Wilson’s disease, the diagnosis is made when the serum ceruloplasmin seems to be low, and the copper concentrations result in high urinary copper levels, when there are suggestive brain MRI abnormalities, and Kayser–Fleisher rings where there are copper depositions in the periphery of the cornea seen during the slit lamp examination. The brain MRI features are diffuse and symmetrical T2-weighted hyperintensities in the striatum, thalamus, brainstem, cerebellum, and possibly white matter. Treatment consists of a low copper diet and copper chelating agents such as D-penicillamine and trientine or zinc, which interfere with the absorption of copper. Liver transplantation could be considered a rescue option in patients with severe neurological forms (albeit without severe necrotic lesions) that are resistant to anti-copper therapies [143][47].References
- Young, R.R.; Shahani, B.T. Asterixis: One Type of Negative Myoclonus. Adv. Neurol. 1986, 43, 137–156.
- Ellul, M.A.; Cross, T.J.; Larner, A.J. Asterixis. Pract. Neurol. 2017, 17, 60–62.
- Gokula, R.M.; Khasnis, A. Asterixis. J. Postgrad. Med. 2003, 49, 272–275.
- Agarwal, R.; Baid, R. Asterixis. J. Postgrad. Med. 2016, 62, 115–117.
- Pal, G.; Lin, M.; Laureno, R. Asterixis-History and Terminology. Neurology 2015, 84, S44.004.
- Adams, R.D.; Foley, J.M. The Neurological Changes in the More Common Types of Severe Liver Disease. Trans. Am. Neurol. Assoc. 1949, 74, 217–219.
- Pal, G.; Lin, M.M.; Laureno, R. Asterixis: A Study of 103 Patients. Metab. Brain Dis. 2014, 29, 813–824.
- Young, R.R.; Shahani, B.T. Anticonvulsant Asterixis. Electroencephalogr. Clin. Neurophysiol. 1973, 34, 760a.
- Inoue, M.; Kojima, Y.; Mima, T.; Sawamoto, N.; Matsuhashi, M.; Fumuro, T.; Kinboshi, M.; Koganemaru, S.; Kanda, M.; Shibasaki, H. Pathophysiology of Unilateral Asterixis Due to Thalamic Lesion. Clin. Neurophysiol. 2012, 123, 1858–1864.
- Río, J.; Montalbán, J.; Pujadas, F.; Alvarez-Sabín, J.; Rovira, A.; Codina, A. Asterixis Associated with Anatomic Cerebral Lesions: A Study of 45 Cases. Acta Neurol. Scand. 1995, 91, 377–381.
- Nayak, R.; Pandurangi, A.; Bhogale, G.; Patil, N.; Chate, S. Asterixis (Flapping Tremors) as an Outcome of Complex Psychotropic Drug Interaction. J. Neuropsychiatry Clin. Neurosci. 2012, 24, E26–E27.
- Verma, S.K.; Pruthi, S.; Khamesra, R.; Bordia, A. Asterixis in Cerebral Malaria. J. Assoc. Physicians India 1989, 37, 484.
- Foundas, M.; Donaldson, M.D.; McAllister, I.L.; Bridges, L.R. Vision Loss Due to Coincident Ocular and Central Causes in a Patient with Heidenhain Variant Creutzfeldt-Jakob Disease. Age Ageing 2008, 37, 231–232.
- Muneta, S.; Yamashita, Y.; Fukuda, H.; Watanabe, S.; Imamura, Y.; Matsumoto, I. Asterixis and Astatic Seizures in Association with Bilateral Insular Lesions in a Patient with Viral Encephalitis. Intern. Med. 1995, 34, 756–761.
- Kang, J.H.; Im, S. Functional Anatomy of the Spinal Tracts Based on Evolutionary Perspectives. Korean J. Neurotrauma 2023, 19, 275–287.
- Kojovic, M.; Cordivari, C.; Bhatia, K. Myoclonic Disorders: A Practical Approach for Diagnosis and Treatment. Ther. Adv. Neurol. Disord. 2011, 4, 47–62.
- Butz, M.; Timmermann, L.; Gross, J.; Pollok, B.; Südmeyer, M.; Kircheis, G.; Häussinger, D.; Schnitzler, A. Cortical Activation Associated with Asterixis in Manifest Hepatic Encephalopathy. Acta Neurol. Scand. 2014, 130, 260–267.
- Reinfeld, H.; Louis, S. Unilateral Asterixis. Clinical Significance of the Sign. N. Y. State J. Med. 1983, 83, 206–208.
- Adams, R.D.; Foley, J.M. The Neurological Disorder Associated with Liver Disease. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1953, 32, 198–237.
- Ugawa, Y.; Shimpo, T.; Mannen, T. Physiological Analysis of Asterixis: Silent Period Locked Averaging. J. Neurol. Neurosurg. Psychiatry 1989, 52, 89–93.
- Peterson, D.I.; Peterson, G.W. Unilateral Asterixis. Bull. Clin. Neurosci. 1986, 51, 77–80.
- Aravamuthan, B.R.; Waugh, J.L. Incidence and Etiologies of Pediatric Asterixis. Mov. Disord. 2016, 31, 373.
- Santamaría Cano, J.; Graus Ribas, F.; Martínez Matos, J.; Rubio Borrero, F.; Arbizu Urdiain, T.; Peres Serra, J. Asterixis in focal lesions of the nervous system. Rev. Clin. Esp. 1983, 168, 37–39.
- Vallat, J.M.; Rkina, M.; Bokor, J. Unilateral Asterixis Due to Subdural Hematoma. Arch. Neurol. 1981, 38, 535.
- Sunwoo, M.K.; Jang, H.-S.; Roh, S.Y.; Yoo, H.J.; Jeong, E.H.; Kim, B.-S.; Choe, Y.R.; Lee, K.-E. Asterixis in the Leg Induced by Anterior Cerebral Artery Infarction. Neurol. Sci. 2016, 37, 979–981.
- Stell, R.; Davis, S.; Carroll, W.M. Unilateral Asterixis Due to a Lesion of the Ventrolateral Thalamus. J. Neurol. Neurosurg. Psychiatry 1994, 57, 116–118.
- Rissardo, J.P.; Fornari Caprara, A.L. Dystonia and Asterixis in Acute Thalamic Infarct: Proposed Mechanism. Ann. Mov. Disord. 2019, 2, 138–139.
- Donat, J.R. Unilateral Asterixis Due to Thalamic Hemorrhage. Neurology 1980, 30, 83–84.
- Massey, E.W.; Goodman, J.C. Unilateral Asterixis. JAMA 1979, 241, 133–134.
- Gila, L.; García Díaz, J.J.; Campos, C.; Gil Pujades, A.; Otal, M. Unilateral asterixis associated with anatomic cerebral lesions. Rev. Clin. Esp. 1991, 188, 355–357.
- Yagnik, P.; Dhopesh, V. Unilateral Asterixis. Arch. Neurol. 1981, 38, 601–602.
- Feil, K.; Huber, M.; Goldschagg, N.; Kellert, L. Unilateral Asterixis in Arm and Leg Caused by Internal Capsula Stroke. Case Rep. Neurol. Med. 2018, 2018, 3946380.
- Mizutani, T.; Shiozawa, R.; Nozawa, T.; Nozawa, Y. Unilateral Asterixis. J. Neurol. 1990, 237, 480–482.
- Trejo, J.M.; Giménez-Roldán, S.; Esteban, A. Focal Asterixis Caused by a Small Putaminal Hemorrhage. Mov. Disord. 1986, 1, 271–274.
- Song, I.-U.; Kim, J.-S.; An, J.-Y.; Kim, Y.-I.; Lee, K.-S. Co-Occurrence of Astasia and Unilateral Asterixis Caused by Acute Mesencephalic Infarction. Eur. Neurol. 2007, 57, 106–108.
- Bonnaud, I.; Salama, J. An ischemic syndrome of the oculumotor nucleus: Associated clinical and anatomical variations on a theme. Rev. Neurol. (Paris) 2003, 159, 781–785.
- Nerei, R.; Murakami, T.; Kawase, S.; Takigawa, H.; Hanajima, R. . Rinsho Shinkeigaku 2022, 62, 793–796.
- Siniscalchi, A.; Gallelli, L.; Di Benedetto, O.; De Sarro, G. Asterixis as a Presentation of Cerebellar Ischemic Stroke. West. J. Emerg. Med. 2012, 13, 507–508.
- Choi, S.-M. Movement Disorders Following Cerebrovascular Lesions in Cerebellar Circuits. J. Mov. Disord. 2016, 9, 80–88.
- Leavitt, S.; Tyler, H.R. Studies in Asterixis. Arch. Neurol. 1964, 10, 360–368.
- Butterworth, R.F. Neurosteroids in Hepatic Encephalopathy: Novel Insights and New Therapeutic Opportunities. J. Steroid Biochem. Mol. Biol. 2016, 160, 94–97.
- Singhal, A.; Nagarajan, R.; Kumar, R.; Huda, A.; Gupta, R.K.; Thomas, M.A. Magnetic Resonance T2-Relaxometry and 2D L-Correlated Spectroscopy in Patients with Minimal Hepatic Encephalopathy. J. Magn. Reson. Imaging 2009, 30, 1034–1041.
- Frederick, R.T. Current Concepts in the Pathophysiology and Management of Hepatic Encephalopathy. Gastroenterol. Hepatol. 2011, 7, 222–233.
- Eberhardt, O.; Topka, H. Myoclonic Disorders. Brain Sci. 2017, 7, 103.
- Miyata, Y.; Hamanaka, K.; Kumada, S.; Uchino, S.; Yokochi, F.; Taniguchi, M.; Miyatake, S.; Matsumoto, N. An Atypical Case of KMT2B-Related Dystonia Manifesting Asterixis and Effect of Deep Brain Stimulation of the Globus Pallidus. Neurol. Clin. Neurosci. 2020, 8, 36–38.
- Rissardo, J.P.; Vora, N.M.; Tariq, I.; Mujtaba, A.; Caprara, A.L.F. Deep Brain Stimulation for the Management of Refractory Neurological Disorders: A Comprehensive Review. Medicina 2023, 59, 1991.
- Poujois, A.; Sobesky, R.; Meissner, W.G.; Brunet, A.-S.; Broussolle, E.; Laurencin, C.; Lion-François, L.; Guillaud, O.; Lachaux, A.; Maillot, F.; et al. Liver Transplantation as a Rescue Therapy for Severe Neurologic Forms of Wilson Disease. Neurology 2020, 94, e2189–e2202.
More
