Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Alfredo Caturano.
Diabetic gastroparesis (DGP) is a debilitating gastrointestinal disorder characterized by delayed gastric emptying in individuals with diabetes mellitus, particularly type 1 and long-standing type 2 diabetes. This condition, although not widely known, significantly impacts the quality of life of affected individuals. The symptoms of DGP, including early satiety, excessive fullness after meals, loss of appetite, bloating, abdominal pain, and vomiting, stem from the slowed or stalled movement of food from the stomach to the small intestine.
- diabetic gastroparesis
- nutritional intervention
- pathophysiology
- diabetes
1. Diagnosis
Diabetic gastroparesis (DGP) presents a diagnostic challenge, demanding a comprehensive approach for accurate identification and management. The clinical diagnosis begins with a meticulous evaluation of the patient’s medical history, focusing on symptoms such as early satiety, postprandial fullness, nausea, vomiting, and erratic blood glucose control, especially in individuals with a long-standing history of diabetes, particularly those with type 1 or longstanding type 2 diabetes [7,76,77][1][2][3]. A thorough physical examination is crucial, encompassing abdominal assessments to detect signs like distension or tenderness, alongside an evaluation of nutritional status. Exclusion of other conditions that mimic DGP symptoms, such as peptic ulcer disease or intestinal obstruction, involves tests like upper endoscopy, imaging studies, and medication reviews to rule out potential causes. In the evaluation of suspected gastroparesis, it is imperative to exclude mechanical obstructions through diagnostic procedures such as upper endoscopy, computed tomography, or magnetic resonance enterography [78][4]. In cases where no obstructions are detected, specialized gastrointestinal function tests become instrumental in diagnosing disturbances associated with diabetic gastroenteropathy.
Specialized diagnostic tests play a pivotal role in confirming delayed gastric emptying and differentiating DGP from other motility disorders. Gastric scintigraphy (GES), considered the gold standard, evaluates gastric emptying non-invasively by employing a standard low-fat meal, tracking both solid and liquid phases. Delayed gastric emptying is diagnosed if there is greater than 60% retention at 2 h or 10% retention at 4 h. Mild, moderate, and severe classifications are made based on 10–15%, 16–35%, and >35% gastric retention after 4 h, respectively [79][5]. Notably, sex differences impact solid gastric emptying rates, with females being approximately 15% slower than males. GES limitations include variations in protocols across institutions, limited access to gamma-camera facilities, and radiation exposure concerns, restricting its use in specific populations, such as pregnant women or children.
The Gastric Emptying Breath Test (GEBT) offers a noninvasive approach to assess gastric emptying rates. Utilizing a 13C-labeled substrate in a standardized meal, GEBT measures exhaled 13CO2 at intervals (45, 90, 120, 150, 180, and 240 min) [6]. The 13C substrate is absorbed in the duodenum, releasing 13CO2 in breath samples. While GEBT is radiation-free, cost-effective, and suitable for certain populations, it remains an indirect measure of gastric emptying, with individual metabolism variations not fully understood.
The Wireless Motility Capsule (WMC) has served as a safe alternative to GES. This small wireless transmitting capsule records and transmits pH, pressure, and temperature data as it travels through the gastrointestinal tract. Gastric emptying time is determined by a pH shift as the capsule moves from the acidic stomach to the alkaline duodenum. The patient ingests the WMC after a standardized nutrient meal, and normal emptying should occur within 5 h. WMC pressure measurements can distinguish between patients with DGP and healthy individuals, demonstrating fewer contractions and motility indices in the former [80][7]. However, despite its historical significance, the WMC is no longer available.
Additionally, the Ambulatory Motilis 3D-Transit System, although holding promise for advancing our understanding of gastrointestinal motility, is currently not available in clinical practice. This innovative system monitors electromagnetic capsules as they traverse the gastrointestinal tract, furnishing detailed insights into gut contractile activity, movement velocity, orientation, and regional transit times. The system’s capability to provide valuable anatomical information enables a comprehensive analysis of colonic motility, encompassing motor patterns and dynamics [80][7].
Finally, it is important to note that the diagnosis of diabetes presents a complex clinical landscape marked by challenges in establishing a clear association between delayed gastric emptying and symptomatic manifestations [81][8]. While delayed gastric emptying is often considered a hallmark of gastroparesis, the strength of its correlation with the daily symptoms experienced by individuals with diabetes mellitus remains elusive [82][9]. This diagnostic uncertainty is further compounded by the observation that a significant number of patients with delayed gastric emptying are asymptomatic, and that symptom profiles in diabetes mellitus patients with normal or delayed gastric emptying often exhibit striking similarities [83,84][10][11]. Moreover, therapeutic trials utilizing prokinetic agents for gastroparesis have revealed a lack of consistent correlation between improvements in gastric emptying and symptom relief [85][12]. In this context, exploring the intricacies of the diagnostic process becomes imperative, shedding light on the limitations of existing techniques and emphasizing the importance of considering subjective symptoms alongside objective measures during gastric emptying studies for a more nuanced and accurate diagnosis. Despite its traditional role in diagnosis, delayed gastric emptying suggests a potentially stronger relevance to glycemia and, conceivably, in guiding therapeutic interventions [1,86][13][14]. Our evolving understanding prompts a nuanced perspective that goes beyond the conventional view of delayed gastric emptying as the hallmark feature.
2. Effects of Diabetic Gastroparesis on Glycemia
The gastrointestinal tract plays a crucial role in glucose homeostasis, with gastric emptying rates influencing blood glucose concentrations. While fasting blood glucose levels are regulated by insulin and glucagon secretion, hepatic and peripheral glucose uptake, and postprandial glucose levels are influenced by various factors. These factors include glucose absorption, disposal, endogenous glucose production, meal composition, gastric emptying rate, small intestine processes (such as glucose absorption and incretin release), insulin secretion, and hepatic and peripheral glucose disposal [90,91,92][15][16][17]. The gastric emptying rate accounts for about 35% of the initial rise in postprandial glucose levels, contributing significantly to both early and overall postprandial glycemia [93,94][18][19]. Notably, patients with gastroparesis in Type 1 diabetes require reduced early postprandial insulin compared to those with a normal gastric emptying rate [95][20]. In intervention studies with Type 2 diabetes patients, inhibiting gastric emptying with opiates markedly decreased glycemic excursions, while prokinetic erythromycin administration had opposite effects [96][21]. Understanding the relationship between slowing gastric emptying and reductions in glycemia is crucial. Precise evaluation using intraduodenal glucose infusions spanning physiological rates demonstrated a nonlinear glycemic response, emphasizing the significance of even modest changes in intestinal glucose flux. Moreover, studies indicate that accelerated gastric emptying leads to postprandial hyperglycemia, while abnormally delayed emptying might predispose individuals to hypoglycemia. Therefore, measuring gastric emptying in insulin-treated diabetes patients with unexplained hypoglycemic episodes, particularly in the early postprandial period, is vital. Adjustments in insulin regimens or therapies to enhance gastric emptying predictability, typically by accelerating it, should be considered based on these measurements [97,98][22][23].3. Nutritional Management of Diabetic Gastroparesis
DGP is a clinical condition characterized by an increased risk of malnutrition, both in quantitative and qualitative terms. Data from The NIDDK Gastroparesis Clinical Research Consortium (GpCRC) in 2011 revealed that more than 60% of the 305 patients enrolled in the study experienced malnutrition, failing to meet the minimum required levels of calories, and many of them presented severe vitamin deficiencies. This registry encompasses all patients with gastroparesis, regardless of the presence of type 1 or type 2 diabetes. Particularly in type 2 diabetic individuals, the data showed a significant number of patients who were overweight, contrary to the common idea that identifies underweight as the sole hallmark of malnutrition. According to the study results, only a very small percentage of gastroparetic patients received nutrition counseling from a qualified dietitian or followed a suggested diet to minimize gastrointestinal discomfort [99][24]. All these data underscore the crucial importance of nutritional assessment in all individuals affected by gastroparesis, with special attention to diabetic individuals due to implications for glucose management. In the absence of evidence-based guidelines, nutritional management of DGP relies on expert recommendations, observational studies, and clinical consensus. Initially, all patients diagnosed with DGP should undergo an assessment for malnutrition. A BMI < 20 kg/m2 or unintentional weight loss of 5–10% of body weight within the last 3–6 months are considered severe clinical markers of malnutrition [100,101][25][26]. Being overweight, as discussed below, does not exclude the presence of a risk or actual malnutrition. In diabetic individuals, the presence of early morning satiety could be an indirect sign of gastroparesis. To avoid excluding overweight individuals from nutritional surveillance, a weight goal, above which clinical intervention must be performed, should be identified for all individuals affected by DGP, leading to a specific nutritional plan tailored to each patient. One of the general recommendations is to avoid large meals. For individuals with DGP, six or eight meals during the day are suggested. Large meals, indeed, both slow gastric emptying and reduce lower esophageal sphincter pressure. Food consistency is crucial. Unlike solids, liquid meals do not require antral contraction to pass through the stomach, emptying simply by gravity. Moreover, well-prepared liquid meals can be highly caloric. If feasible, transitioning from solid to liquid meals could be suggested for all gastroparetic patients. Consuming liquid meals in the later part of the day could alleviate gastroparetic symptoms. Parrish et al. suggest in their work a specific semi-liquid meal pattern combining liquid foods and liquid supplements. Moreover, they suggest enhancing the protein and caloric load, flavor, and palatability of liquid foods [102][27]. Experts also recommend avoiding a supine position in the first hours after a meal, as antigravity effects and duodenal compression by the spine could immediately worsen gastroparetic symptoms. For the same reason, elevating the head by 6–8 cm during sleep is suggested to minimize reflux. Patients with DGP are prone to the formation of bezoars due to their incapability to properly eliminate fibers. Generally, fibers are contraindicated in patients with DGP, although their possible effect in minimizing the glycemic index of foods is recognized. Some researchers are exploring technological solutions to improve gastric tolerance to fibers in gastroparetic individuals [103][28]. Suresh et al. have recently demonstrated that some fibers characterized by low viscosity (PHGG—partially hydrolyzed guar gum or Arabic gum) are better tolerated in terms of gastrointestinal symptoms than high viscosity ones (psyllium husk) in people affected by DGP presenting the same effects in mitigating glycemic index. These results seem encouraging in a new definition of the role of fibers in DGP [103][28]. Fats, in general, reduce gastric emptying, although they may be better tolerated in liquid form. Limiting their consumption to general dietary recommendations in people with diabetes, based on the patients’ gastric tolerance, could be encouraged. Another aspect to consider is that hyperglycemia (glycemia > 200 mg/dL) itself inhibits gastric emptying. Trying to avoid glycemic variability and adapting insulin administration following a basal-bolus regimen to the nutritional habits of the patient affected by DGP is a fundamental goal for diabetologists, also aiming to improve the quality of life and reduce gastric symptoms in patients. To date, there are no recommendations for choosing specific insulin analogs over others. In summary, the main nutritional suggestions for diabetic individuals experiencing gastroparesis are as follows (Figure 1) [102][27]: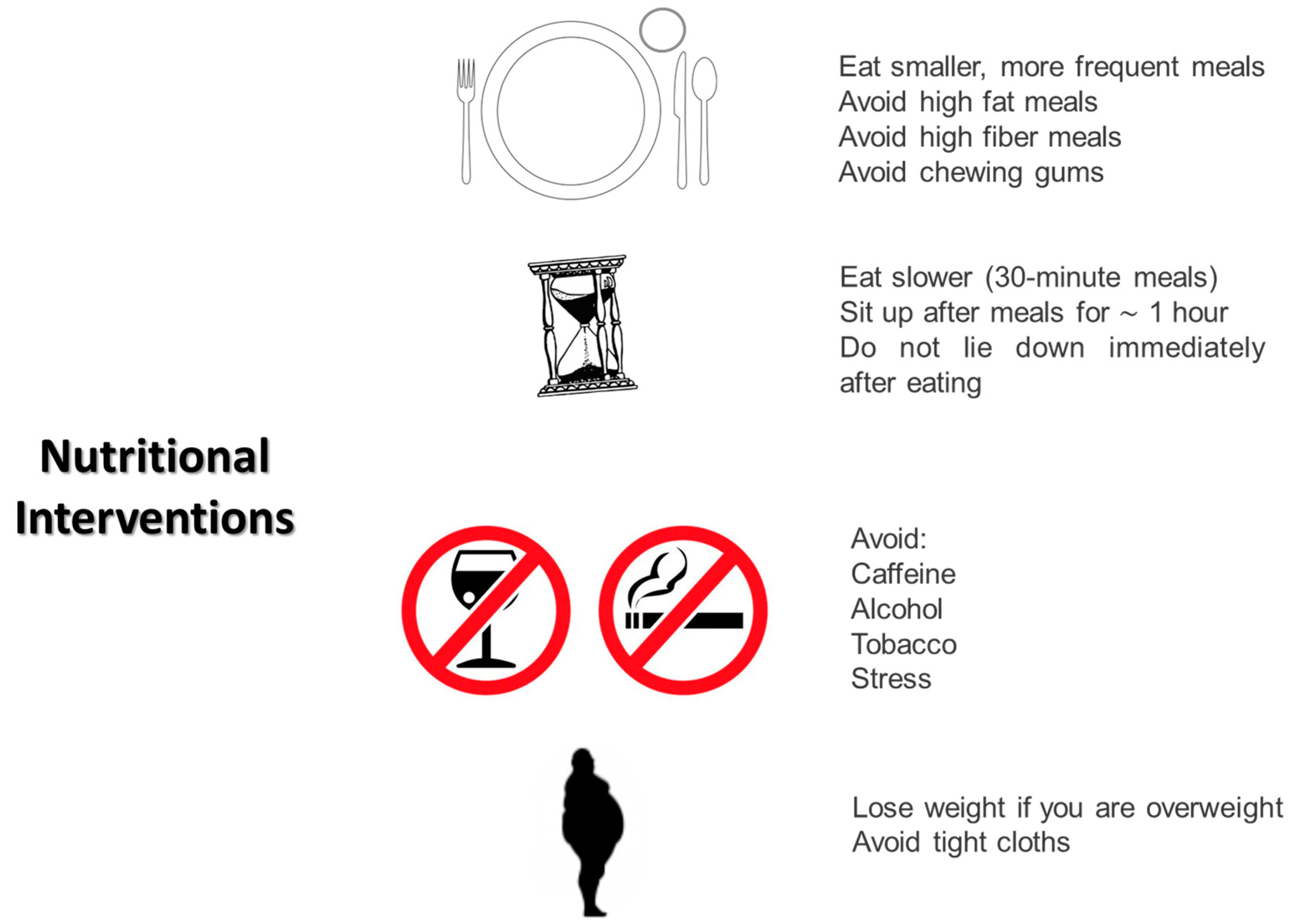
Figure 1.
Main nutritional interventions in patients with diabetic gastroparesis.
-
Eating smaller and more frequent meals;
-
Eating slowly (30 min meals);
-
Avoiding the supine position for at least the first hour after a meal;
-
Sleeping with the head elevated 6–8 inches from the bed to minimize reflux;
-
Avoiding tight clothes that could compress the abdomen;
-
Avoiding meals in the later part of the day;
-
Avoiding fats and fibers;
-
Avoiding chewing gums that increase air swallowing;
-
Avoiding CATS: caffeine, alcohol, tobacco, and stress;
-
Avoiding all foods that can reduce lower esophageal sphincter pressure: peppermint, chocolate, fat, and caffeine.
References
- Petri, M.; Singh, I.; Baker, C.; Underkofler, C.; Rasouli, N. Diabetic gastroparesis: An overview of pathogenesis, clinical presentation and novel therapies, with a focus on ghrelin receptor agonists. J. Diabetes Complicat. 2021, 35, 107733.
- Farmer, A.D.; Kadirkamanathan, S.S.; Aziz, Q. Diabetic gastroparesis: Pathophysiology, evaluation and management. Br. J. Hosp. Med. 2012, 73, 451–456.
- Camilleri, M.; Iturrino, J.; Bharucha, A.E.; Burton, D.; Shin, A.; Jeong, I.D.; Zinsmeister, A.R. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol. Motil. 2012, 24, 1076-e562.
- Parkman, H.P.; Hasler, W.L.; Fisher, R.S.; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004, 127, 1592–1622.
- Young, C.F.; Moussa, M.; Shubrook, J.H. Diabetic Gastroparesis: A Review. Diabetes Spectr. 2020, 33, 290–297.
- Pafundi, P.C.; Garofalo, C.; Galiero, R.; Borrelli, S.; Caturano, A.; Rinaldi, L.; Provenzano, M.; Salvatore, T.; De Nicola, L.; Minutolo, R.; et al. Role of Albuminuria in Detecting Cardio-Renal Risk and Outcome in Diabetic Subjects. Diagnostics 2021, 11, 290.
- Meldgaard, T.; Keller, J.; Olesen, A.E.; Olesen, S.S.; Krogh, K.; Borre, M.; Farmer, A.; Brock, B.; Brock, C.; Drewes, A.M. Pathophysiology and management of diabetic gastroenteropathy. Therap. Adv. Gastroenterol. 2019, 12, 1–17.
- Vijayvargiya, P.; Jameie-Oskooei, S.; Camilleri, M.; Chedid, V.; Erwin, P.J.; Murad, M.H. Association between delayed gastric emptying and upper gastrointestinal symptoms: A systematic review and meta-analysis. Gut 2019, 68, 804–813.
- Teigland, T.; Iversen, M.M.; Sangnes, D.A.; Dimcevski, G.; Søfteland, E. A longitudinal study on patients with diabetes and symptoms of gastroparesis—Associations with impaired quality of life and increased depressive and anxiety symptoms. J Diabetes Complicat. 2018, 32, 89–94.
- Freeman, R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014, 126, 63–79.
- Khayyam, U.; Sachdeva, P.; Gomez, J.; Ramzan, Z.; Smith, M.S.; Maurer, A.H.; Fisher, R.S.; Parkman, H.P. Assessment of symptoms during gastric emptying scintigraphy to correlate symptoms to delayed gastric emptying. Neurogastroenterol. Motil. 2010, 22, 539–545.
- Janssen, P.; Harris, M.S.; Jones, M.; Masaoka, T.; Farré, R.; Törnblom, H.; Van Oudenhove, L.; Simrén, M.; Tack, J. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am. J. Gastroenterol. 2013, 108, 1382–1391.
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic Gastroparesis. Endocr. Rev. 2019, 40, 1318–1352.
- Caturano, A.; Galiero, R.; Pafundi, P.C. Metformin for Type 2 Diabetes. JAMA 2019, 322, 1312.
- Woerle, H.J.; Albrecht, M.; Linke, R.; Zschau, S.; Neumann, C.; Nicolaus, M.; Gerich, J.; Göke, B.; Schirra, J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E103–E109.
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135.
- Woerle, H.J.; Meyer, C.; Dostou, J.M.; Gosmanov, N.R.; Islam, N.; Popa, E.; Wittlin, S.D.; Welle, S.L.; Gerich, J.E. Pathways for glucose disposal after meal ingestion in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E716–E725.
- Horowitz, M.; Edelbroek, M.A.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862.
- Jones, K.L.; Horowitz, M.; Carney, B.I.; Wishart, J.M.; Guha, S.; Green, L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J. Nucl. Med. 1996, 37, 1643–1648.
- Ishii, M.; Nakamura, T.; Kasai, F.; Onuma, T.; Baba, T.; Takebe, K. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care 1994, 17, 901–903.
- Gonlachanvit, S.; Hsu, C.W.; Boden, G.H.; Knight, L.C.; Maurer, A.H.; Fisher, R.S.; Parkman, H.P. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig. Dis. Sci. 2003, 48, 488–497.
- Perano, S.J.; Rayner, C.K.; Kritas, S.; Horowitz, M.; Donaghue, K.; Mpundu-Kaambwa, C.; Giles, L.; Couper, J.J. Gastric emptying is more rapid in adolescents with type 1 diabetes and impacts on postprandial glycemia. J. Clin. Endocrinol. Metab. 2015, 100, 2248–2253.
- Rayner, C.K.; Horowitz, M. New management approaches for gastroparesis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 454–493.
- Parkman, H.P.; Yates, K.P.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Calles, J.; Koch, K.L.; Abell, T.L.; et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011, 141, 486–498.
- Sadiya, A. Nutritional therapy for the management of diabetic gastroparesis: Clinical review. Diabetes Metab. Syndr. Obes. 2012, 5, 329–335.
- Di Francia, R.; Rinaldi, L.; Cillo, M.; Varriale, E.; Facchini, G.; D’Aniello, C.; Marotta, G.; Berretta, M. Antioxidant diet and genotyping as tools for the prevention of liver disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5155–5163.
- Parrish, C.S.; Pastors, J.G. Nutritional Management of Gastroparesis in People with Diabetes. Diabetes Spectr. 2007, 20, 231–234.
- Suresh, H.; Zhou, J.; Ho, V. The Short-Term Effects and Tolerability of Low-Viscosity Soluble Fibre on Gastroparesis Patients: A Pilot Clinical Intervention Study. Nutrients 2021, 13, 4298.
More
