Prostate cancer accounts for a significant proportion of cancer diagnoses in Canadian men. Approximately one-third of all prostate cancers are metastatic at the time of diagnosis (synchronous) or recur (metachronous) following definitive treatment. Over the past decade, the therapeutic landscape for the management of metastatic prostate cancer has undergone rapid changes. Novel strategies use hormonal agents, chemotherapy, homologous recombination repair inhibitors, and radioligand therapy or combination strategies in addition to androgen deprivation therapy. In this review, we summarize the available data addressing key therapeutic areas along the disease continuum and focus on practical aspects for general practitioners in oncology managing patients with metastatic prostate cancer
- prostate cancer
- ARAT
- androgen receptor axis-targeted drugs
- docetaxel
- BRCA1/2
- breast cancer gene 1/2
1. Introduction
2. Metastatic Castrate-Sensitive Prostate Cancer
The benefits of surgical or pharmacologic castration with androgen deprivation therapy (ADT) were established in 1941 [12]. Metastatic prostate cancer was consequently considered to be either castration-sensitive or -resistant, referring to an early state of the disease susceptible to castration and a later state that is no longer vulnerable to testosterone suppression, respectively. A paradigm shift occurred in 2015 with the advent of combination therapy, so-called “intensification beyond androgen deprivation therapy”. Improved overall survival (OS) was demonstrated in studies assessing the efficacy of adding docetaxel or androgen biosynthesis inhibition with abiraterone acetate and prednisone along with blockade of the androgen receptor with enzalutamide or apalutamide. These data will be summarized below.A. Cytotoxic Drug Therapy
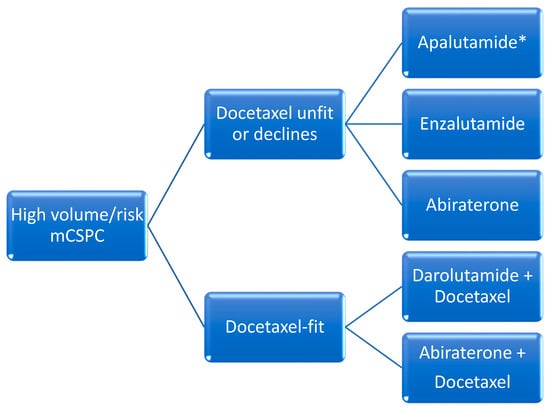
The addition of docetaxel was the first agent to demonstrate favorable efficacy in this setting. When compared to ADT alone, docetaxel administered over six cycles every 21-days initiated within the first 120 days of ADT in patients with de novo metastatic disease was associated with improved median OS in patients with high-volume disease (median OS 44 months with combination vs 32.2 months with ADT alone). High-volume disease was defined as the presence of visceral metastasis or at least four bone metastases of which at least one had to be present outside of the spine and pelvis [13]. All other disease states with metastases that do not meet these criteria are referred to as low-volume. In 2016, another trial confirmed these results, with an improvement in median OS of 10 months [14] (Figure 1 and Figure 2).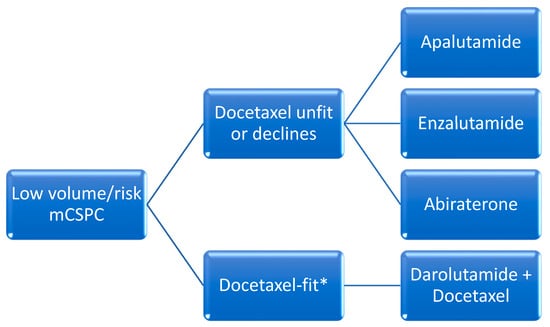
B. Oral Hormonal Agents and ADT (“Doublet-Therapy”)
Abiraterone acetate demonstrated an OS benefit when administered until disease progression in patients with de novo metastatic disease and high-risk features defined as two of the following: Gleason score 8 or higher, visceral disease and at least three bone metastases. Radiographic progression free survival (rPFS) was 33 months in the abiraterone group versus 14.8 months with placebo. The reduction in the risk of radiologic progression was 53%. Median OS was 53.3 months in patients receiving abiraterone versus 36.5 months in patients receiving placebo, with a relative improvement in OS of 33% [7]. Enzalutamide was associated with improved survival in patients with de novo metastatic disease in the ARCHES trial [16]. The primary endpoint, median rPFS, was not reached with enzalutamide plus ADT versus 19 months with placebo plus ADT, with a significant reduction in risk of 61%. The risk reduction for death in the final report was significantly decreased by 33%. In the ENZAMET trial, patients randomized to ADT and enzalutamide versus ADT and non-steroidal antiandrogens demonstrated a survival advantage that favored enzalutamide [17][18]. Median OS was not reached after a median follow-up period of 44 months in ARCHES and 68 months in ENZAMET. A subgroup analysis of ENZAMET patients who had received prior docetaxel chemotherapy did not demonstrate any incremental OS benefit. Apalutamide was assessed in the TITAN trial, which randomized patients with mCSPC to ADT plus apalutamide versus placebo plus ADT and primary endpoint of rPFS. In this study, about 10% of patients had received prior docetaxel chemotherapy. Furthermore, about one-third of patients did not have high-volume disease using the CHAARTED definition. Investigators showed that rPFS was improved by 52% in the apalutamide group whereas OS was improved by 35% and median OS was not reached for the apalutamide group versus 52.2 months in patients receiving ADT alone [19][20]. A novel second-generation drug unavailable in North America is rezvilutamide. This compound’s efficacy and safety were assessed in the phase III CHART trial, which enrolled men with high-volume mCSPC who had never received chemotherapy and randomized them to ADT plus rezvilutamide versus ADT plus bicalutamide. Notably, 90% of patients were Chinese. Improved rPFS was demonstrated with rezvilutamide compared to bicalutamide (median rPFS not reached) versus 25.1 months (HR 0.44 [95% CI 0.33–0.58); p < 0.0001). Rezvilutamide significantly improved overall survival compared to bicalutamide (HR 0.58 (95% CI 0.44–0.77); p = 0.0001), whereas median OS was not reached for either arm at the time of publication. The most common grade ≥3 adverse events were hypertension (8% versus 7%), hypertriglyceridemia (7% versus 2%), weight gain (6% versus 4%), anemia (4% versus 5%) and hypokalemia (3% versus 1%), with rezvilutamide compared to bicalutamide, respectively [21].C. Docetaxel with Oral Hormonal Agents and ADT (“Triplet-Therapy”)
As of 2024, none of these strategies have been formally compared to one another in a clinical trial and are considered alternatives. The most recent development in this setting is the combination of ADT, docetaxel chemotherapy and either abiraterone or darolutamide.i. Darolutamide with Docetaxel and ADT
ARASENS was a phase III trial that assessed the efficacy and safety of darolutamide in patients with mCSPC who were candidates for ADT and docetaxel. Participants were randomized to ADT and six cycles of docetaxel and darolutamide versus ADT, docetaxel and placebo. The primary endpoint was OS and was not reached for the darolutamide group versus 48.9 months for placebo, with a significant improvement in the risk of death of 32%. The study did not stratify patients by risk or volume of disease [22]. A subsequent report assessed therapeutic benefits in all volume and risk settings, and concluded that patients with high-volume mCSPC were most likely to profit from triplet therapy whereas patients with except for low-volume mCSPC were most likely to benefit from ADT plus androgen receptor-targeted agents.disease [23].ii. Abiraterone with Docetaxel and ADT
Abiraterone was assessed in the PEACE-1 trial which compared ADT, docetaxel and abiraterone vs ADT and docetaxel in patients with de novo metastatic hormone sensitive prostate cancer. Patients were stratified by disease volume as per the CHAARTED criteria mentioned above. Participants with high-volume disease had significant improvement in OS (median 61 vs. 42 months), whereas patients with low-volume did not [24]. Achievement of undetectable 8-month PSA after initiation of ADT with abiraterone and docetaxel predicted improvement in rPFS and OS [25].iii. Triplet Therapy Data Limitations
Given the lack of available head-to-head comparisons between triplet (darolutamide or abiraterone with docetaxel and ADT) and doublet strategies (ARAT and ADT), the use of either approach is currently accepted as a reasonable evidence-based alternative. In all cases, a careful discussion of the available evidence is recommended to enable patients to make well-informed decisions. A systematic review of all the main phase III trials indirectly compared these different approaches and concluded that the best OS and radiologic PFS occurred with abiraterone-based triplet therapy vs. ADT alone, notwithstanding study limitations. However, this is still an indirect comparison of clinical trials with different methodologies and disease characteristics such as high and low volume and/or risk, and the information must therefore be interpreted with caution [23].D. Radiotherapy for Low-Volume Disease
The role of radiotherapy in newly diagnosed metastatic prostate cancer was investigated in the STAMPEDE trial, which compared systemic therapy alone to systemic therapy plus radiotherapy to the prostate using 55 Gy in 20 fractions or 36 Gy in 6 fractions. Systemic therapy consisted of lifelong ADT with or without docetaxel (18%). Metastatic burden was defined per the CHAARTED criteria above. In patients with low-burden disease, but not high-burden, radiotherapy improved 5-year overall survival (HR 0.64, 95% CI 0.52–0.79; p < 0.001), failure-free survival and progression free survival [26][27]. These data suggest that prostate radiotherapy should be recommended as a standard of care for these men.3. Putting It All Together: Practical Recommendations for Clinicians
Most men with metastatic prostate cancer are candidates for intensification above androgen deprivation. Important considerations when prescribing and supervising ARAT agents include understanding efficacy (summarized above), addressing relevant toxicities, managing drug–drug interactions, determining progressive disease and understanding unique considerations for older adults (Figure 3). This section introduces the latter four concepts. In all cases, the standard recommendation is to monitor drug therapy on a continuous basis.
4. Conclusions
Promising new therapeutic strategies for men with metastatic prostate cancer are improving outcomes and have manageable toxicity profiles. Clinicians are encouraged to develop their knowledge and experience using androgen receptor axis-targeted agents as well as managing relevant toxicities in collaboration with medical oncologists. Recommendations from the Canadian Urologic Association, American Urologic Association and European Society of Medical Oncology are available online and are useful resources. Collaborative efforts that include multi-disciplinary cancer clinicians are needed to optimize care for men with incurable prostate cancer.
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. J. De L’association Medicale Can. 2022, 194, E601–E607.
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021, 79, 243–262.
- Debes, J.D.; Tindall, D.J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1488–1490.
- Cookson, M.S.; Roth, B.J.; Dahm, P.; Engstrom, C.; Freedland, S.J.; Hussain, M.; Lin, D.W.; Lowrance, W.T.; Murad, M.H.; Oh, W.K.; et al. Castration-resistant prostate cancer: AUA Guideline. J. Urol. 2013, 190, 429–438.
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005.
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351.
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360.
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433.
- 9. Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148.
- 10. Marchioni, M.; Di Nicola, M.; Primiceri, G.; Novara, G.; Castellan, P.; Paul, A.K.; Veccia, A.; Autorino, R.; Cindolo, L.; Schips, L. New Antiandrogen Compounds Compared to Docetaxel for Metastatic Hormone Sensitive Prostate Cancer: Results from a Network Meta-Analysis. J. Urol. 2020, 203, 751–759.
- Mateo, J.; Fizazi, K.; Gillessen, S.; Heidenreich, A.; Perez-Lopez, R.; Oyen, W.J.G.; Shore, N.; Smith, M.; Sweeney, C.; Tombal, B.; et al. Managing Nonmetastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 285–293.
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J. Urol. 2002, 168, 9–12.
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746.
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177.
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, 1616–1622.
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986.
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131.
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Cheung, L.; Chi, K.N.; Chowdhury, S.; Frydenberg, M.; Horvath, L.G.; Joshua, A.M.; et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 323–334.
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303.
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24.
- Gu, W.; Han, W.; Luo, H.; Zhou, F.; He, D.; Ma, L.; Guo, H.; Liang, C.; Chong, T.; Jiang, J.; et al. Rezvilutamide versus bicalutamide in combination with androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer (CHART): A randomised, open-label, phase 3 trial. Lancet. Oncol. 2022, 23, 1249–1260.
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142.
- Jian, T.; Zhan, Y.; Yu, Y.; Yu, K.; Hu, R.; Wang, J.; Lu, J. Combination therapy for high-volume versus low-volume metastatic hormone-sensitive prostate cancer: A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1148021.
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707.
- 1361MO—8-Month PSA Strongly Predicts Outcomes of Men with Metastatic Castration-Sensitive Prostate Cancer in the PEACE-1 Phase III Trial. Gravis Mescam. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2022/8-month-psa-strongly-predicts-outcomes-of-men-with-metastatic-castration-sensitive-prostate-cancer-in-the-peace-1-phase-iii-trial (accessed on 24 January 2020).
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Ali, A.; Amos, C.L.; Attard, G.; Chowdhury, S.; Cook, A.; Cross, W.; et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022, 19, e1003998.
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Amos, C.L.; Attard, G.; Chowdhury, S.; Cook, A.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366.
- Potdar, R.; Gartrell, B.A.; Given, R.; Karsh, L.; Frankel, J.; Nenno, K.; O’MalleyLeFebvre, K.; Bhaumik, A.; McCarthy, S.; McGowan, T.; et al. Concomitant use of oral anticoagulants in patients with advanced prostate cancer receiving apalutamide: A post-hoc analysis of TITAN and SPARTAN studies. Am. J. Cancer Res. 2022, 12, 445–450.
- Watanabe, A.; Momo, K.; Tanaka, K.; Kida, M.; Kuchira, R.; Koshizuka, H.; Nishimura, K.; Morita, M.; Yamamoto, M.; Sasaki, T. Drug-drug interaction between apalutamide and atorvastatin through breast cancer resistance protein in an outpatient. Int. J. Clin. Pharmacol. Ther. 2022, 60, 367–369.
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418.
- Boyle, H.J.; Alibhai, S.; Decoster, L.; Efstathiou, E.; Fizazi, K.; Mottet, N.; Oudard, S.; Payne, H.; Prentice, M.; Puts, M.; et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur. J. Cancer 2019, 116, 116–136.
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708.
- Jayadevappa, R.; Chhatre, S.; Malkowicz, S.B.; Parikh, R.B.; Guzzo, T.; Wein, A.J. Association Between Androgen Deprivation Therapy Use and Diagnosis of Dementia in Men with Prostate Cancer. JAMA Netw. Open 2019, 2, e196562.
- Nead, K.T.; Gaskin, G.; Chester, C.; Swisher-McClure, S.; Dudley, J.T.; Leeper, N.J.; Shah, N.H. Androgen Deprivation Therapy and Future Alzheimer’s Disease Risk. J. Clin. Oncol. 2016, 34, 566–571.
- Dinh, K.T.; Reznor, G.; Muralidhar, V.; Mahal, B.A.; Nezolosky, M.D.; Choueiri, T.K.; Hoffman, K.E.; Hu, J.C.; Sweeney, C.J.; Trinh, Q.D.; et al. Association of Androgen Deprivation Therapy with Depression in Localized Prostate Cancer. J. Clin. Oncol. 2016, 34, 1905–1912.
- Gonzalez, B.D.; Jim, H.S.L.; Booth-Jones, M.; Small, B.J.; Sutton, S.K.; Lin, H.-Y.; Park, J.Y.; Spiess, P.E.; Fishman, M.N.; Jacobsen, P.B. Course and Predictors of Cognitive Function in Patients with Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison. J. Clin. Oncol. 2015, 33, 2021–2027.
- Batra, A.; Marchioni, M.; Hashmi, A.Z.; Lonergan, P.E.; Morgans, A.K.; Nead, K.T.; Nguyen, P.L.; Winquist, E.; Chin, J.L. Cognition and depression effects of androgen receptor axis-targeted drugs in men with prostate cancer: A systematic review. J. Geriatr. Oncol. 2021, 12, 687–695.
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699.
- Hinz, A.; Mehnert, A.; Kocalevent, R.-D.; Brähler, E.; Forkmann, T.; Singer, S.; Schulte, T. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 2016, 16, 22.
