PBM may aid in repairing axonal damage by increasing the production of cellular energy currency, adenosine triphosphate (ATP). This process involves the regulation of various secondary mediators: reactive oxygen species (ROS), nitric oxide (N), cyclic adenosine monophosphate (cAMP, and calcium ions (Ca
2+). These mediators play key roles in cellular signaling and function, and their modulation by PBM can lead to the activation of pathways that promote the regeneration of axons
[10][11].
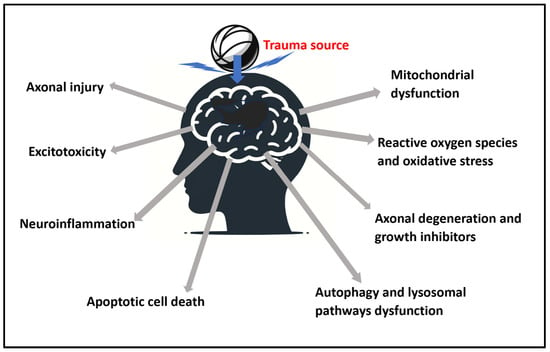
2.2. Mitochondrial Dysfunction
The functions of mitochondria include oxidative phosphorylation, where oxygen is used to produce ATP. They also have important roles in ion homeostasis, several metabolic pathways, apoptosis and programed cell death, and ROS production and consumption
[11][13]. The role of these physiological aspects, and the need to balance them are expanded below. The dysfunction of the mitochondria in managing these are linked to many neurological conditions
[12][14]. At the cellular level, the main cause of secondary harmful cascades is cell damage centered in the mitochondria
[13][15].
Electron microscopy studies of mitochondria post-TBI have shown significant structural damage, such as swelling, disruption of cristae (internal structures of mitochondria), and loss of membrane potential, all of which indicate impaired mitochondrial function. In the process, proteins such as cytochrome c and apoptosis-inducing factor (AIF) are released into the cytosol, leading to further cell death
[14][15][16,17].
PBM’s effectiveness is often attributed to its action on mitochondria, particularly cytochrome c oxidase (CCO) in the electron transport chain (ETC), playing a key role in enhancing oxidative phosphorylation
[16][18]. It is hypothesized that inhibitory nitric oxide can be dissociated from CCO. This is part of the process of PBM enhancing ETC activity, mitochondrial membrane potential, and promoting cellular recovery
[17][19].
2.3. Excitotoxicity
The blood–brain barrier (BBB) is a protective barrier that regulates the movement of substances between the bloodstream and the brain. TBI-induced BBB breakdown leads to excessive excitatory amino acid release, particularly glutamate, resulting in oxidative stress and prolonged excitotoxicity
[18][20].
Excitotoxicity occurs when glutamate over-activates its receptors, such as N-methyl-D-aspartate (NMDA) and kainate (KA) receptors, leading to an influx of calcium ions (Ca
2+) into neurons. Excessive glutamate during TBI is also attributed to a failure of glutamate re-uptake due to the dysfunction of glutamate transporters
[19][21]. The excess Ca
2+ can trigger a cascade of harmful events, including oxidative stress and neuronal damage
[18][20].
While studies show PBM increases Ca
2+ concentration in healthy cells, PBM also plays a homeostatic role in moderating the toxic levels of intracellular Ca
2+ content in stressed cells. In an in vitro study, excitotoxicity was induced in cultured cortical neurons with glutamate, N-methyl-D-aspartate (NMDA), and kainic acid (KA) to produce 50% cytotoxicity. The effects of PBM on these excitotoxic cells were compared against a control of healthy neurons
2.4. Reactive Oxygen Species, Reactive Nitrogen Species, and Oxidative Stress
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated as natural byproducts of the normal metabolism of oxygen. At low levels, ROS is essential for the regulation of physiological cellular functions, including cellular signaling, differentiation, and survival
[20][21][23,24]. RNS has been recognized as playing a crucial role in the physiologic regulation of many, if not all, living cells, including nervous system cells
[22][25].
TBI causes an overproduction of ROS and RNS which can overwhelm the brain’s antioxidant defenses. This leads to oxidative stress, a state where the body’s ability to detoxify these excess reactive products is disrupted. Oxidative stress results in damage to cell components, including lipids, proteins, and DNA. This can cause mutations, malfunctions in cellular signaling pathways, and can initiate programmed cell death (apoptosis), contributing to chronic traumatic encephalopathy (CTE) neurodegeneration and other diseases
[23][24][26,27].
PBM generally produces ROS at low levels, resulting in positive effects
[25][28]. PBM has been shown to modulate ROS levels positively, increasing antioxidant enzyme activity in superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), leading to the reduction of oxidative stress in brain tissues
[26][27][29,30]. Injured cells subjected to high levels of oxidative stress, such as in the case of TBI, are also more responsive to PBM.
Near infrared (NIR) PBM has been found to regulate the production of ATP and ROS in mitochondria. Interestingly, in isolated mitochondria, a 980 nm diode laser wavelength at 100 mW decreases ATP and increases ROS production, whereas 800 mW and above increases ATP and reduces ROS production
[28][33]. The relatively higher power was shown to be more inhibitive to ROS.
2.5. Neuroinflammation
In response to TBI, glial cells, including microglia and astrocytes, become activated and release inflammatory mediators, contributing to the inflammatory response
[29][36]. TBI can lead to a breakdown or disruption of the BBB, further facilitating the infiltration of immune cells and substances that can exacerbate inflammation and neuronal damage
[30][37] Excessive or prolonged glial activation can cause neurotoxicity, synaptic dysfunction, and neurodegeneration
[31][38].
A study published in 2023 used a mouse model to investigate inflammation induced by lipopolysaccharide (LPS), a component found in the outer membrane of certain bacteria known to trigger an immune response. The study demonstrated PBM’s efficacy in downregulating proinflammatory cytokines (IL-1β and IL-18). These cytokines are signaling molecules that promote inflammation and are typically elevated after TBI. In the meantime, PBM was also shown to upregulate anti-inflammatory cytokines (IL-10). These molecules help in reducing inflammation and promoting healing.
2.6. Axonal Degeneration and Growth Inhibitors
In TBI, axons, which are responsible for transmitting information in the nervous system, can become damaged or severed. This damage can lead to axonal degeneration, disrupts neural communication, and can be responsible for brain swelling and neuronal death
[32][41]. Axonal degeneration can trigger post-traumatic neurodegeneration, presented with toxic protein pathologies amyloid and hyperphosphorylated tau. These protein pathologies are found in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. In the case of TBI, its equivalent is the presence of CTE
[33][34][42,43].
After TBI, the brain can produce molecules known as growth inhibitors
[4]. These molecules impede the ability of neurons to regenerate axons, thus hindering the repair process. Glial cells, such as astrocytes and microglia, may contribute to this process by forming a scar tissue barrier around the injury site, which releases inhibitory molecules preventing axonal regrowth. They can also activate downstream effectors that lead to axonal collapse
[35][44].
PBM has been shown to promote axonal regeneration. This could be due to PBM’s effects on mitochondrial function in neurons, enhancing their energy production and survival, which are crucial for repair and regeneration processes
[10][11].
2.7. Apoptotic Cell Death
TBI can trigger a form of programmed cell death, apoptosis, in both neurons and glial cells. This process involves a series of biochemical events leading to characteristic cell changes and death. It involves mitochondrial dysfunction, caspase activation, DNA fragmentation, and phagocytosis
[36][47]. The loss of neurons and glial cells through apoptosis significantly contributes to the loss of brain function. It can also induce secondary inflammatory cascades that can exacerbate brain damage
[37][48].
PBM has shown potential in reversing the apoptotic process. This effect is believed to be mediated through its action on cellular mitochondria
[10][11]. PBM can activate cell survival pathways involving various signaling molecules and proteins that work together to prevent the cell from undergoing apoptosis
[17][19].
In addition to its role in inhibiting cell death, PBM has also been observed to stimulate neurogenesis, the process of generating new neurons from neural stem cells. An animal study demonstrated that PBM can enhance neurogenesis following injuries similar to ischemic stroke. The proposed mechanisms underlying this effect include (1) the promotion of proliferation and differentiation of neural progenitor cells within the peri-infarct zone, which is the area surrounding the damaged brain tissue; and (2) the improvement of the neuronal microenvironment. This improvement is achieved by modulating the inflammatory status and enhancing mitochondrial function
[38][49].
Angiogenesis plays a crucial role in supporting neurogenesis during the recovery from TBI. Specifically, angiogenesis, stimulated by vascular endothelial growth factor (VEGF), has been shown to significantly enhance neurogenesis and reduce lesion volumes post-TBI
[39][50]. This process is also critical in remodeling neurogenesis following ischemic strokes
[40][51]. PBM has demonstrated efficacy in promoting angiogenesis. It does this by modulating endothelial dysfunction
[41][52] and aiding in wound healing
[42][53], serving as examples of its angiogenic effects.
2.8. Autophagy and Lysosomal Pathways Dysfunction
Autophagy is a cellular process that involves the degradation and recycling of damaged or unnecessary cellular components. It plays a crucial role in maintaining cellular homeostasis and health. Lysosomes are cellular organelles that contain enzymes to break down waste materials and cellular debris and are integral to the autophagy process. Autophagy and lysosomal function are essential for maintaining cellular homeostasis, clearing damaged or unwanted materials, and regulating cellular metabolism
[43][58].
TBI can disrupt these processes, impairing the ability of cells to efficiently remove and recycle damaged components. This disruption can lead to the accumulation of toxic proteins and cellular components, contributing to cell death and exacerbating brain damage
[44][45][59,60].
PBM is hypothesized to regulate ROS levels, thereby facilitating healthy mitophagy and maintaining cellular homeostasis. Mitophagy is a specific type of autophagy focused on the degradation and recycling of mitochondria. It helps to support the brain which is impaired during TBI
[46][61] as a result of mitochondrial dysfunction
[13][15]. By regulating ROS levels, PBM may enhance mitophagy, helping cells to remove dysfunctional mitochondria
[47][62]. This in turn supports protein activity (involving PINK1/Parkin signaling)
[48][63], which helps regulate mitophagy
[49][64].
3. Additional Relevant Systemic and Secondary PBM Mechanisms
3.1. Increased Cellular Energy Production
In PBM, when photons from the light source interact with cytochrome c oxidase in mitochondria, it can lead to increased ATP production. This enhanced energy production improves cellular function and repairs damaged brain tissues
[50][71].
3.2. Enhanced Blood Flow and Oxygenation
PBM is believed to enhance cellular energy availability by improving blood circulation through the photodissociation of nitric oxide (NO). This improves blood flow and oxygen delivery to the injured brain region. It promotes tissue repair and reduces hypoxic conditions that can exacerbate TBI-related damage
[51][65]. A 2016 published animal study suggested that 660 and 810 nm wavelengths pulsing at 10 Hz produced the best outcomes in TBI by improving blood flow and oxygenation
[52][66].
3.3. Modulation of Synaptic Plasticity
PBM may influence synaptic plasticity, which is the ability of synapses to strengthen or weaken over time, affecting neuronal signaling. By promoting synaptic plasticity, PBM could enhance cognitive recovery and functional improvements in TBI patients
[53][54][67,68].
The above literature on the effects of PBM on the pathophysiology of TBI shows the promise of PBM for treating TBI. The real value will lie in the translation to human use, as confirmed by clinical study data.
3.4. Effect on Ferroptosis
Ferroptosis can play a significant role in neuronal death and brain damage following the injury
[55][72]. It is a form of regulated cell death characterized by iron-dependent lipid peroxidation linked to oxidative stress and inflammation
[56][69]. PBM has been observed to reduce oxidative stress
[57][70] and modulate inflammatory responses
[58][39], which could influence ferroptosis pathways.
4. New Discoveries in Cellular Mechanisms Inform Future PBM Treatments
4.1. Increase in Cellular Current Flow and Resistance
One of the characteristic features of a living cell is that it controls the exchange of electrically charged ions across the cell membrane
[59][85]. PBM has been shown to allow more electrical current to flow through cells. Interestingly, PBM also in the meantime, increases cellular resistance or resilience, which is important for the functional integrity of the myelin sheaths of the axons. This was achieved with 810 nm wavelength delivered at 10 Hz
[60][86]. Further investigation to explore the characteristics of other pulse frequencies is warranted.
4.2. Polymerization of Tubulins
Dimers of α- and β-tubulin polymerize to form microtubules, which are composed of 13 protofilaments assembled around a hollow core. Tubulin dimers can depolymerize as well as polymerize, and microtubules can undergo rapid cycles of assembly and disassembly
[61][87]. PBM pulsed at 10 Hz at 810 nm demonstrated depolymerization of tubulins
[60][86]. There is also evidence that intervention with this set of parameters can destabilize the secondary structure of the microtubules, with α-helices transitioning into β sheets
[62][88]. As microtubules are a core component of neuronal integrity, more knowledge in this area has implications for TBI recovery and avoidance of CTE neurodegenerative progression.
4.3. The Significance of Pulse Frequency
More recent studies extend beyond the effects of PBM on molecular mechanisms—they offer clues that each parameter such as wavelength, power density, and pulsing rates could influence physiological outcomes. This hypothesis is supported by other investigations that have shown that pulse frequencies influence the brain response.
As an example, in 2019, Zomorrodi et al. published for the first time that inducing a certain PBM frequency in the brain can change the waveforms of the brain. The study delivered 810 nm wavelength at 40 Hz (gamma) to the hubs of the default mode network (DMN) of healthy brains and found that this increases the power of the faster oscillations of alpha, beta, and gamma, while reducing the power of the slower oscillations of delta and theta
[63][83].
5. Summary
Transcranial PBM represents a multifaceted therapeutic intervention for TBI. PBM's full potential in treating TBI is in its early stages. Intensifying research efforts could significantly advance the knowledge of the optimal parameters, thereby enhancing treatment outcomes.