Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Khalid Sossey-Alaoui and Version 2 by Lindsay Dong.
Breast cancer comes in different types, making it hard to treat effectively. One particularly aggressive type, called triple-negative breast cancer, is tough to target with current treatments. Scientists use advanced methods like 3D cultures, which mimic human tissue better than traditional lab methods, to study breast cancer. These 3D cultures help understand how tiny communication structures called exosomes affect cancer growth, spread, and response to therapy. Exosomes are like messengers between cells and can influence cancer’s behavior and response to therapy.
- exosomes
- 3D culture
- breast cancer
- TNBC
- organoids
- extracellular vesicles
- exosomes
1. Introduction
Breast cancer, the foremost cause of cancer-related deaths globally, exhibits distinct subtypes with variations in pathology, genetics, and clinical features, predominantly affecting women [1]. Despite advancements in breast cancer treatment methods, its histological and functional heterogeneity poses a significant clinical challenge. Current evidence indicates that these subtypes manifest diverse genetic alterations, where cell adhesion and secreted extracellular proteins play crucial roles in shaping the tumor microenvironment [2][3][2,3]. The identification of targeted therapies for breast cancer based on genomic alterations remains challenging due to extensive molecular heterogeneity, particularly in metastatic cases [4]. Triple-negative breast cancer (TNBC), characterized by aggressiveness and metastatic potential, presents additional challenges due to the lack of targeted therapeutics [5].
Maintaining cancer heterogeneity in culture is vital for understanding genotype–phenotype relationships, influencing the success of targeted agents. In recent years, advanced 3D culture technologies have provided more representative models of both healthy human tissue and various malignancies [6][7][6,7]. Organoids and spheroids, intricate three-dimensional structures resembling organs, have been instrumental in advancing cancer research [8]. Sato and his team pioneered the development of 3D epithelial organoids, setting the stage for organoid development from various human epithelial tissues, including the colon, prostate, stomach, liver, lungs, pancreas, esophagus, endometrium, taste buds, salivary glands, fallopian tubes, and breast tissue [9].
In cancer studies, 3D cultures serve as intermediate models between 2D cultures and in vivo studies, offering a more accurate representation of in vivo tumors’ characteristics. This includes aspects such as cell-to-cell and cell-to-extracellular matrix contacts, cellular layering, hypoxia, and gradients of nutrients and pH [10][11][10,11]. Evaluating extracellular vesicles (EVs) in vitro through 3D culture systems provides distinct advantages over in vivo assessments. Despite the challenges associated with monitoring EVs in vivo, a considerable amount of research has focused on this approach [12][13][14][12,13,14].
Poor prognosis in breast cancer is linked to rapid tumor progression, interactions within the tumor microenvironment, and the dissemination of tumor cells through blood circulation to form metastatic tumors in other organs [15]. Studies have shown that tumor-derived extracellular vesicles, specifically exosomes (50–150 nm diameter), released from cells can transfer bioactive molecules to neighboring or distant cells via body fluids such as the bloodstream [16]. Due to their unique trafficking characteristics, exosomes are emerging as potential therapeutic RNA delivery vehicles for early diagnosis, prognosis, and targeted therapy development [17][18][19][17,18,19]. The process of harvesting exosomes is influenced by the culture system, isolation method, and purification process employed [20][21][20,21]. Accumulating evidence suggests that a three-dimensional (3D) culture system yields more exosomes compared to the traditional two-dimensional (2D) system [22]. Moreover, exosomes produced by 3D culture exhibit enhanced therapeutic effects through the transfer of specific cargoes, emphasizing their potential in clinical cancer studies [19].
2. Exosomes in 2D versus 3D Culture System
In recent decades, an expanding body of cancer studies has brought to light that exosomes, small nano-sized molecules ranging from 30 to 1020 nm, represent the primary class of extracellular vesicles secreted by breast cancer and stromal/cancer-associated fibroblast cells into the extracellular milieu and tumor microenvironment [23]. Under stressful conditions, exosomes released by breast cancer cells have been linked to an increase in the invasive and metastatic potential of these cells [24]. The unique biogenesis of exosomes involves distinct intracellular regulatory processes that equip them with specific cargos and, consequently, diverse biological functions. Fibroblast-derived exosomes, for example, have been demonstrated to enhance breast cancer metastasis and motility via the Wnt pathway [25]. Mesenchymal stem cell (MSC) exosomes have displayed remarkable efficacy in cancer metastasis, tissue repair, and regeneration across various organs, including the liver, lungs, cartilage, myocardium, brain, spinal cord, kidney, and breast [26][27][28][29][30][26,27,28,29,30]. In investigating the tumor microenvironment and cell-to-cell communications, the conventional in vitro method is the 2D culture, a “gold standard” approach for cultivating monolayer cells and isolating exosomes. This choice is primarily supported by its simplicity, reproducibility, and cost effectiveness. However, several studies have indicated that the 2D model, in terms of mechanical and biochemical features, falls short of accurately mimicking the pathophysiology and features of three-dimensional (3D) confirmation with specific architecture found in in vivo solid tumors [10]. Conventional 2D cell culture methods particularly struggle to replicate the density and heterogeneity of clinical tumors as observed in vivo. Consequently, over recent years, alternative 3D culture models have been under development. The shift to 3D culture environments aims to address the disparities between monolayer cells under 2D conditions and the in vivo conditions where cells grow in three dimensions, impacting cell morphology, cell signaling crosstalk, interactions with the extracellular matrix (ECM), drug screening, and the understanding of tumor progression, invasion, and metastasis [14][31][32][33][34][14,31,32,33,34]. Lee and colleagues established the most suitable 3D cell culture methods for studying stem cells and the microenvironment of breast cancer, developing a 3D culture system for the mammary epithelium that enables the generation and long-term expansion of three-dimensional epithelial organoids [35]. When compared to 2D cultures, 3D culture environments revealed altered dynamics and molecular contents of breast cancer exosome secretion, including RNA [36]. The findings from the study suggest that exosomal RNA content derived from 3D culture could replicate in vivo tissue-derived exosomal RNAs. Studies have identified microRNAs that are more prevalent in 3D-culture-derived cervical cancer exosomes compared to those derived from 2D culture [14]. In the 3D cell culture system, interactions between cells and the extracellular environment closely resemble those in tumor tissue masses, as depicted in Figure 1.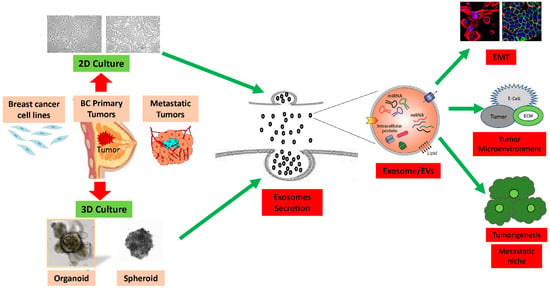
Figure 1. Summary of 2D vs. 3D cultures derived from breast cancer cell lines and tumors: The efficiency of production of exosomes and extracellular vesicle (EVs) are significantly higher in the 3D culture models compared to the 2D culture models. Exosomes contain integrins and other cellular proteins, lipids, nucleic acids, as well as miRNAs, which they transfer into the extracellular microenvironment. Exosomes and other extracellular vesicle cargo induce immune system suppressive pathways due to interaction with extracellular matrix to promote epithelial-to-mesenchymal transition (EMT), breast cancer tumorigenesis, invasion, and metastasis.
3. Exosomes and 3D Culture Organoid Model
One significant stride in cancer stem cell research over the past decade has been the emergence of “organs” known as organoids. In the realm of classical developmental cancer biology studies, “organoid” refers to a self-organizing 3D structure derived from stem cells [37][47]. Organoids, which faithfully mimic the in vivo architecture and cell differentiation of the original tissue in mammals, utilize defined developmental cues. The phenotypes of stem cells can be differentiated using specific conditional media [38][48]. Three-dimensional organoid culture systems are extensively employed as a disease model in studies, encompassing the expansion of tumor-initiating cells [39][49], investigation into invasion, progression, and metastatic developments, as well as drug screening to understand cell responses to irradiation [40][50].
While the value of 3D cell culture in studying tumor cells is well established, Rocha and colleagues showed that tumor cells cultured in 3D conditions secrete EVs that contain protein cargo and miRNAs that are distinct from those contained in 2D EVs, emphasizing the need for further investigation into EV biogenesis markers [41][55].
Since its identification, the spheroid model of ovarian cancer has proven to be particularly relevant for in vitro studies, providing insights into how EVs contribute to carcinogenesis through stem cell initiation and differentiation. This system offers enhanced reliability and stability, mirroring in vivo situations more closely [42][56].
4. Exosomes Derived from 3D Culture Improve Therapeutics Effect
The potential impact of exosomes in the field of cancer biotherapy is extensive. Exosomes may also serve as a versatile vehicle for administering cancer treatment. Exosomes play pivotal roles in transferring proteins, nucleic acids, and lipid molecules, positioning them as a promising avenue for effective cancer therapy and targeted antigen/drug carriers. Notably, studies in cancer have revealed that exosomes released from MSCs of various sources differentially influence tumor progression, invasion, and metastasis [43][74]. The therapeutic efficacy of MSC-based therapies, mediated through exosome-mediated paracrine secretion of cytokines and growth factors, has been increasingly demonstrated. A major class of MSC exosomes inherently possesses therapeutic potential due to their unique cargoes, making them a promising framework for cell-based therapies. These exosomes can act as nature’s own delivery tools, serving as biotherapeutics and mimicking the functions of cellular therapies without the side effects of embolism and proliferation. Various strategies are being developed for cancer therapy, including targeting exosomes to inhibit their impact on diseases, manipulating their intrinsic therapeutic potential, and utilizing them for drug delivery. Inhibition of cancer malignancy in vitro has been demonstrated using a clathrin-mediated endocytosis inhibitor, chlorpromazine, targeting the mechanism of exosome miRNA-21 uptake [44][45][46][100,101,102]. While exosomes represent a relatively new field in cancer research, substantial interest exists in their potential application as low-toxicity and targeted inhibitors in immunotherapy, as cancer biomarkers, and for targeted therapy [47][104]. Tumor microenvironments play a crucial role in anti-tumor immunity, offering a newly identified strategy for cancer therapeutics. Understanding the influence of exosomes on components of the tumor microenvironment (TME) is vital for advancing the clinical benefits of tumor microenvironment inhibitors. This necessitates defining the impact of exosomes on various components of the TME using appropriate cancer models, contributing to the discovery of novel therapeutic approaches for breast cancer [48][49][105,106].5. Three-Dimensional Exosomes Regulate Microenvironment in Breast Cancer
Breast cancer metastasis is a highly regulated and a complex process involving local invasion, extravasation, intravasation, transport, and colonization [50][51][107,108]. These stages necessitate key regulators, encompassing genetic, biochemical, and morphological changes within an evolutionarily conserved and interconnected program known as the epithelial-mesenchymal transition (EMT) [52][53][54][55][109,110,111,112]. Cancer extracellular vesicles, including exosomes, play a crucial role in modifying normal epithelial cells, a phenomenon termed EMT, contributing to the initiation and progression of oncogenesis [56][61]. In 3D cultures, exosome secretion is promoted through signal exchange, often occurring within the context of spheroids or organoids. The extracellular matrix (ECM) holds significant importance in both the homeostasis of normal breast tissues and the breast cancer microenvironment, owing to its specific composition, morphology, and cellular changes [57][113]. The ECM, a complex mixture of multicellular regulatory proteins, exhibits specific structural and functional properties [58][114]. Three-dimensional cell culture enhances the physical properties of the ECM with a complex network of extracellular bodies governing cell fate through biochemical transmission and biomechanical mechanisms [59][115]. MSC-derived exosomes have emerged as contributors to the metastatic niche through interactions with the stromal and matrix components, regulating immune responses targeted towards tumoroids [60][116]. Numerous studies have explored various components within the tumor microenvironment present in 3D spheroids, such as tumor-associated endothelial cells (TECs), cancer-associated fibroblasts (CAFs), tumor neovasculature, adipocytes, and immune cells, including tumor-associated macrophages (TAM), cytokines, tumor-infiltrating lymphocytes (TILs), and myeloid-derived suppressor cells (MDSCs) [61][62]. Exosomes have been shown to participate in immune suppression, affecting the regulation of CD8+ T cells, MDSCs, and Tregs [62][117]. Microenvironment heterogeneity is effectively replicated in a 3D system, such as the secretion of miRNA-21-enriched exosomes by glioblastoma stem-like cells, regulating the miR-21/VEGF/VEGFR2 signaling pathway, and promoting permeability and angiogenesis in the glioma microenvironment [63][64][120,121]. CSC-derived exosomes facilitate bidirectional crosstalk between CSCs and their metastatic niche, promoting microenvironment remodeling, resulting in tumor aggressiveness and distant metastasis [65][122]. These studies identify pre-metastatic niche construction as an underlying mechanism for creating a favorable environment for cancer stem cell growth.6. The Impact of Tumor Derived Exosomes on Immune Suppression and Cancer Progression
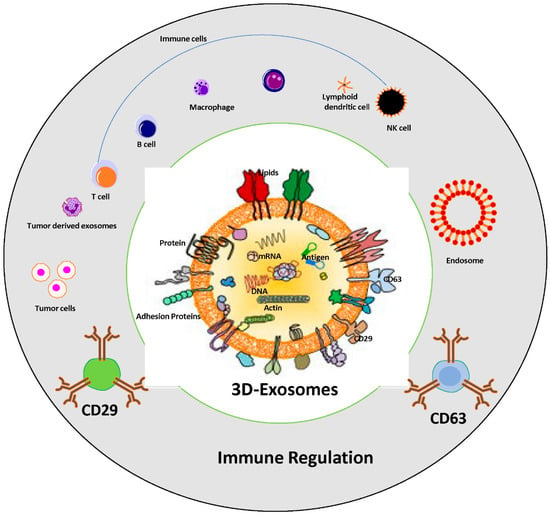
Exosomes possess the ability to suppress the immune system, as cancer cells release these vesicles containing molecules that impede the body’s capacity to recognize and combat cancerous cells. Cancer cells modify the microenvironment and regulate the effect of the functionality of immune system, usually via pathways involving cell-to-cell communication and the release of many suppressive factors [66][67][127,128]. Within these exosomes, proteins capable of inhibiting immune cell activity, particularly T cells, are found. Moreover, exosomes carry microRNAs that regulate the expression of genes involved in immune functions are summarized in Figure 2.
Figure 2. Role of tumor-derived exosomes in immune suppression and cancer progression. Schematic representation of immune regulation system via exosomes; structure of tumor derived exosomes which carry several components, nucleic acids, lipid bilayers and proteins including tetraspanin family, adhesion proteins, antigen binding proteins, etc., are associated with immune cells regulation. The exosomes cargo CD63 and CD29 used as “exosome markers” in cancer progression.
Furthermore, exosomes disrupt the maturation of immature myeloid cells (IMCs) into dendritic cells and monocytes, resulting in an increased population of myeloid-derived suppressor cells (MDSCs) and other immune cells, such as B cells, monocytes can crosstalk with CAFs as well that possessing potent immunosuppressive activity [68][69][70][79,134,135]. These exosomes also exhibit the ability to inhibit the activity of various immune cells, including T cells, B cells, and natural killer (NK) cells [71][72][136,137]. They achieve this by containing proteins such as programmed death-ligand 1 (PD-L1), which interact with receptors on immune cells, suppressing their activity. Moreover, exosomes carry microRNAs regulating the expression of genes involved in immune function [73][138].
7. Exosomes Mediated Multidrug Resistance
Maintaining the integrity of exosomes is crucial for cancer cellular activity and intercellular communication, presenting a significant breakthrough in addressing challenges related to multidrug resistance in cancer management. Breast cancer poses a substantial hurdle to effective treatment due to the complexity of genetic alterations and the high heterogeneity of the disease [74][142]. The mechanisms underlying the invasive, metastatic, and aggressive ability of drug resistance remain elusive [75][76][143,144]. Over the past decade, the development of 3D models for various cancer studies, with a focus on understanding cancer stemness, aggressive behavior, tumorigenesis, and chemoresistance, has significantly expanded our comprehension of these processes [76][144]. Recent findings highlight the functional role of drug resistant-derived exosomal EphA2 in promoting the transmission of an aggressive phenotype and metastasis between breast cancer cells and other cellular components. Increased EphA2 protein expression in drug-resistant cancer cell-derived exosomes serves as a potential prognostic marker for drug resistance-induced breast cancer progression [77][145]. Exosomal miRNA-100-5P induced cisplatin drug resistance in various cancer cells, altering mTOR expression levels [78][146]. Liver and lung cancer induced 5-FU drug resistance through miRNA-32-5P via the AKT pathway, while breast cancer exhibited Adriamycin drug resistance delivered by miRNA-222 and colon cancer demonstrated Cetuximab drug resistance mediated by the AKT/PTEN pathway [79][80][81][82][147,148,149,150]. Breast cancer-derived exosomes containing p-glycoproteins confer chemoresistance to more sensitive recipient cells via MDR proteins, including MDR3, PABP4, and endophilin-a2 [83][151]. ABC transporter G2 (ABCG2), a breast cancer drug resistance protein, serves as a cancer stem cell marker [84][152]. Exosomes, also known as EVs, play specific key roles in the development of multidrug resistance and the transfer of genetic signals that regulate tumorigenesis, intercellular communication, and the extracellular matrix, including immune cell responses [68][79].
In summary, the role of exosomes in the development of multidrug resistance between the tumor microenvironment (TME) and cancer cells is significant. TME, a heterogeneous population of cells in breast cancer, includes cancer-associated fibroblasts, endothelial cells, and immune cells, all interlinked with cytokines and chemokines. Breast cancer exosomes act as carriers of information, facilitating intermediate communication between cancer cells and other cellular matrices, leading to the acquisition of drug resistance [85][165]. With an understanding of drug resistance mechanisms via exosomes, which are loaded with various miRNAs and cargo proteins holding unique features leading to chemoresistance, researchers aim to overcome this challenge in TNBC [86][166].
