You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mutali Junior Musa and Version 2 by Wendy Huang.
Systemic lupus erythematosus (SLE) is a chronic autoimmune systemic disorder of the connective tissue that does not affect only one certain organ but has a diversified effect all over the body. The etiology of SLE is may be idiopathic, genetic, hormonal, or environmental. The diagnosis of SLE is difficult and requires a series of tests and findings which can be systemic or ocular. Approximately one-third of those diagnosed with SLE experience ocular manifestations that range from mild to severe sight-threatening conditions. Ocular manifestations of SLE include keratoconjunctivitis sicca, uveitis, and posterior segment pathologic signs.
- systemic lupus erythematosus
- ocular manifestations
- keratoconjunctivitis sicca
- dry eyes disease
- cornea
- orbit
- extraocular muscles
- posterior segment
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune systemic disorder of the connective tissue that does not affect only one certain organ but has a diversified effect all over the body [1][2][1,2]. The etiology of SLE is may be idiopathic, genetic, hormonal, or environmental [3]. Choudhary et al. have suggested that SLE is nine times more common in females as compared to males [4]. SLE may either be of juvenile-onset or adult-onset [5]. In its early stages, both hereditary and acquired immune systems are implicated, leading to the activation of T and B cells. The result is an overproduction of pro-inflammatory cytokines [3]. The diagnosis of SLE is difficult and requires a series of tests and findings which can be systemic or ocular. Approximately one-third of those diagnosed with SLE experience ocular manifestations that range from mild to severe sight-threatening conditions. Ocular manifestations of SLE include keratoconjunctivitis sicca, uveitis, and posterior segment pathologic signs [6]. It has been found that symptoms of SLE in the eye, such as pathological changes in the posterior segment, and other symptoms that may be observed systemically aid in diagnosis as well as treatment for SLE [7].
The involvement of the orbital tissue in SLE is rare and includes the inflammation of the orbit, myositis (infarction of orbital muscles), exophthalmos, panniculitis, subcutaneous orbital tissue inflammation, and orbital edema. Common symptoms include ophthalmoplegia, reduced vision, ocular proptosis, eyelid lesions, and orbital inflammation [1][8][1,8]. Elevated levels of creatine kinase, aldolase, CRP, procalcitonin, and myoglobins are reported from laboratory test results [1][2][3][4][5][6][7][8][9][1,2,3,4,5,6,7,8,9].
Dry eye syndrome is commonplace in SLE [10]. Dry eye associated with SLE may be caused by abnormalities of the lacrimal gland, lacrimal duct, conjunctiva, cornea, and meibomian glands, and encompasses both aqueous-deficient and evaporative dry eye [11]. Patients with SLE have been found to present with severe dry eye symptoms, which might enable the diagnosis of dry eye as an indicator for diagnosing SLE [12]. Reduced corneal sensitivity is a hallmark of severe dry eye that may alter the patient’s perception of ocular discomfort symptoms [13]. Reduced ocular sensation may offer insufficient feedback to the central nervous system via the ophthalmic nerve, leading to less efferent stimulation to the lacrimal gland, decreased tear production, and the encouragement of a vicious cycle [14].
Scaly, pigmented lesions and a cheek rash of the skin of the eyelids and adnexa have been found to be associated with SLE. Common signs noticed on the eyelid and skin in SLE are chronic erythema of the eyelids [15]. Also, unilateral and bilateral blepharitis can occur, and lesions of the eyelids appear as lumps or concretions that attach strongly to the overlying skin [16]. A complication of the SLE involvement in the eyelid margin is madarosis, which can be difficult to manage [17]. The accumulation of immunoglobulins in the tissues may result in episcleritis in SLE patients. The classic symptom of this is the dilation of the superficial blood vessels which shrink significantly when phenylephrine is applied topically. The worsening of vision and severe pain in the eyes are not common [18]. Periorbital inflammation together with edema are signs that patients with SLE present with. Circumbulbar swelling is a typical sign of dermatomyositis—approximately 5% of people with SLE present with periorbital heliotropic edema [19].
Secondary to keratoconjunctivitis sicca, ulcerative and infective keratitis may occur [20]. Corneal symptoms, which can emerge as the first or second manifestations of SLE, have a variety of clinical characteristics and may be a pointer to an existing inflammatory process [21][22][21,22]. The management of patients with SLE is a multi-specialist approach with rheumatologists, nephrologists, ophthalmologists, optometrists, and other specialists of organs that may be affected due to SLE [23][24][23,24].
Systemic lupus erythematosus, subacute lupus erythematosus, and chronic lupus erythematosus, of which discoid lupus is a subset, constitute the various types of the lupus erythematosus spectrum of disease [25][78]. Clinical presentations are diverse, ranging from mild dry eye syndrome to more severe conditions like retinal vasculitis and optic neuritis [26][79]. Variable ocular involvement arises from immune-mediated processes affecting various ocular structures, including the conjunctiva, cornea, lacrimal gland, EOMs, sclera, the vascular tonic, and the optic nerve. SLE patients are more likely to visit the eye clinic compared to other patients due to its multipronged symptomatology [27][28][80,81]. Yazici et al. reported altered corneal biomechanics in SLE patients as compared to a control group [29][82]. They advocated that these should be taken into consideration while taking measurements. Zhang et al. also assessed a cohort of 93,345 patients, reporting that corneal hysteresis was positively correlated with SLE [30][83]. Genetics may also be a confounding factor in antiphospholipid syndrome (APS) in the course of SLE [31][32][84,85]. Modrzejewska et al. reported on an isolated case of a neonate with pre-retinal hemorrhage secondary to possible vessel thrombosis, whose mother was a known SLE patient [33][86].
2. Anterior Segment
Ocular surface disorders are commonly associated with other chronic inflammatory conditions, more so, keratoconjunctivitis sicca or dry eyes disease constitute the most ocular manifestation in lupus erythematosus [34][35][87,88] and severity may correlate with SLE activity [36][89]. Resch et al. reported a significant increase in corneal Langerhans cells in SLE patients compared to normal patients [37][90]. Generally, the pathophysiological process of dry eyes disease in chronic inflammatory disease such as SLE involves the cellular infiltration of the sebaceous and serous glands involved in tears production and tears integrity maintenance, and a dysregulation of pro-inflammatory cytokine expression [38][39][40][41][91,92,93,94]. The pathogenesis of evaporative dry eye in SLE patients entails excessive inflammatory cellular infiltration of the meibomian gland, atrophy, and vascular enrichment around the meibomian gland [42][43][95,96]. Keratoconjunctivitis sicca has been known to be characterized by an unbridled production of pro-inflammatory cytokines which eventually leads to incipient or manifest chronic ocular surface inflammatory changes [44][45][97,98]. T-helper type 17 lymphocyte (Th-17), which is typified by the production of interleukin-17 (IL-17), a powerful pro-inflammatory mediator, has been specifically identified as the leading cause of chronic ocular surface inflammation and consequent epithelial metaplasia resulting in the loss of goblet cells, the mucin-aqueous adherence complex and subsequent dry eye disease [46][47][48][99,100,101]. A neural origin of excessive cytokine expression by leukocytes due to a failure of the sensory afferent mechanotransduction of mechanosensitive ion channel (Piezo1 and Piezo2) has been proposed as the initial cause of events that could result in the activation of the innate and adaptative immune system, which eventually ends in the dysregulation of autoantigen production and consequent dry eyes disease [3].3. Cornea and Conjunctiva
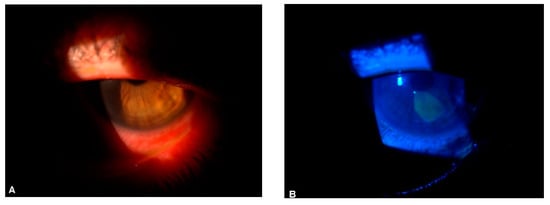
Cornea involvement in SLE may be heralded by chronic poorly managed or severe keratoconjunctivitis sicca and excessive pro-inflammatory cytokine expression. Hyperosmolar tears in SLE patients [49][102] may result in high osmotic desiccating stress on the cornea and conjunctival epithelia surface with a subsequent loss of ocular surface epithelia integrity, distorted epithelial metabolism, chronic ocular surface inflammation, epithelial defect (as shown in Figure 1A,B), cornea ulceration, neovascularization, and scarring. Furthermore, the increased apoptosis of ocular surface epithelia cells in SLE [50][103] may predispose to a higher risk of cornea erosion and keratitis. Recurrent erosive keratopathy, interstitial keratitis [51][104], kerato-endotheliitis [52][105], and peripheral ulcerative keratitis [53][54][73,106] can manifest in SLE as well as other rheumatologic conditions. Interspecialty management between the ophthalmologist, rheumatologist, and other health care is needed.
Figure 1.
(
A
) SLE-linked corneal epitheliopathy in an adult male; (
B
) Same eye, stained with fluorescein sodium and viewed with a cobalt blue filter.
4. Orbit, Extraocular Muscles and Refractive Shift
The involvement of the orbit and extraocular muscles in SLE is rare [36][89] and is usually a diagnosis of exclusion. The manifestations of orbital involvement, such as orbital apex syndrome [55][107], panniculitis [56][108], and orbital vasculitis [57][109], carry a poor prognosis due to associated optic neuropathy. Clinical features include the limitation of gaze, pain, relative afferent pupillary defect, periorbital edema, conjunctival edema, diplopia, and proptosis [58][110]. A relative shift of the habitual refractive status in SLE which is termed “acute onset myopia” has been reported, and it is mostly characterized by an increment of the net dioptric power of the eye. The myopic shift in SLE is proposed to result mostly from ciliary spasm secondary to the ciliary body inflammatory response to autoimmune antibodies and freely circulating cytokines [59][60][111,112]. Subcutaneous fat inflammation, which is known as panniculitis or lupus erythematosus profundus [61][113], is a rare ocular finding in discoid lupus erythematosus and is usually associated with enophthalmos due to subcutaneous lipo-atrophic changes and shrinkage [62][114]. Lupus panniculitis is a refractory disease and early diagnosis is most often difficult and may be confused with panniculitis-like T-cell lymphoma [56][108]. Orbital vasculitis in SLE results in the inflammation of blood vessels that supply the orbit and the orbital viscera with consequent hypo- or nonperfusion of the orbit and its structures. Orbital ischemic attack due to orbital vasculitis could result in an irreversible loss of vision and neovascular glaucoma secondary to ischemic optic neuropathy and retinal ischemia, respectively [63][115]. Orbital myositis in SLE is an immune-related inflammatory activity involving the extraocular muscles with a female preponderance [64][116]. Although rare, bilateral sequential tracheitis has also been reported [65][117]. Signs and symptoms may include restricted gaze, pain in eye movement diplopia, headache, periorbital edema, and ocular hypertension [64][116]. It is usually misdiagnosed as orbital cellulitis, as a diagnosis of exclusion a thorough serologic screening, adequate ophthalmic evaluation ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI) scan are usually required. CT/MRI may demonstrate enlargement and thickening of inflamed extraocular muscles. Treatment is usually with corticosteroid therapy [66][118].5. Uvea and Posterior Segment
The uvea comprises highly vascularized ocular tissues, and its involvement in SLE is rare [67][119]. Posterior segment manifestations can arise from immuno-complex-induced vasculitis secondary to freely circulating anti-cytoplasmic and anti-nuclear antibodies, hypercoagulable states [68][120], infection due to treatment-induced immunosuppression, drug toxicities from pharmaceutical agents used in management [69][70][71][121,122,123], and hematological disorders [67][119]. Posterior segment involvement in SLE includes uveitis [72][124], occlusive retinal vasculitis, optic nerve edema, combined venous and retinal occlusive disease [73][125], central retinal artery occlusion [74][126] macular edema, autoimmune retinopathy [74][126], retinal and vitreous hemorrhages, exudative retinal detachment, posterior scleritis, optic and retinal neovascular membrane, microangiopathy, tortuous retinal vessels, and perifoveal microvasculature abnormalities [75][76][77][127,128,129]. Lupus vasculitis usually involves small and medium blood vessels, whereas the involvement of larger vessels is mostly rare [78][130]. Posterior segment involvement resulting from vasculitis carries a poor prognosis [79][80][131,132]. The pathophysiology of lupus vasculitis is poorly understood. However, a multiplex interaction involving the complement system, autoantibody, and antigen immune complex; which eventually results in polymorphonuclear leukocytes being unleashed on the vascular endothelium and the recruitment of various immune-related inflammatory moieties has been proposed. This immune-related chronic pro-inflammatory event eventually becomes self-sustained, resulting in the formation of autoantibodies and deposition of immune complexes on the vascular endothelium, in turn leading to inflammation and tissue damage. The binding of autoantibodies to antigens creates a soluble immune complex which is then deposited on the vascular endothelium due to a faulty clearance by the reticuloendothelial system and an increased vascular permeability [78][130]. This is then followed by a complement fixation cascade which leads to the recruitment of polymorphonuclear leukocytes, complement protein depletion from phagocytosis, and inflammatory reaction [81][133]. Although rare, the simultaneous presentation of lupus and anti-neutrophilic cytoplasmic antibody (ANCA) has been noted in several instances [82][83][84][134,135,136]. ANCA binds to proteinase3 (PR3) and myeloperoxidase (MPO) antigen resulting in the adherence of neutrophils to the vascular endothelial cells with subsequent inflammation, vascular endothelial damage, and apoptosis [85][86][87][137,138,139]. Furthermore, other auto-antibodies, such as anti-endothelial cell antibody (AECA), have been proposed to induce endothelial cell activation by increasing the expression of endothelial adhesion molecules, such as intracellular adhesion molecule-1 (ICAM-1) and E-selectin, and to alter cytokine production with the consequent secretion of pro-inflammatory cytokines such as interleukin1 [IL1] and tumor necrosis factor [TNF] [88][140]. This may eventually result in the increased adhesion of leukocytes to the endothelial cell, activation of coagulation, inflammatory endothelial damage, vascular injury, and thrombosis [89][141]. Menet et al. reported that SLE was implicated in antiphospholipid syndrome with varying ocular morbidities [90][91][142,143]. Therefore, freely circulating anti-phospholipid antibodies may bind to the phospholipid fragment of the already injured endothelial cell resulting in various thrombotic cascades and subsequent ophthalmic vaso-occlusive crisis [92][144]. Retinal and choroidal manifestations in SLE indicate the severity of the systemic condition [93][94][145,146]. Retinopathy associated with SLE occurs in approximately 12% of affected individuals, although its incidence is reduced with the use of systemic therapy [94][146]. Mild retinopathy can be asymptomatic and incidental, severe vascular occlusive retinopathy is characterized by vision impairment, distortions, and visual field abnormalities. Retinal neovascularization, retinal detachment, microaneurysms, artery constriction, lesions at arteriovenous junctions, retinal edema, retinal exudation, and multifocal serous retinal detachment or pigment epithelial detachment are common retinopathies presented in SLE [3]. Central serous chorioretinopathy (CSCR) is a rare ocular manifestation of SLE which may be caused by choroidal ischemia and inflammation. CSCR is characterized by localized serous neuroepithelial detachments within and around the macula. Reported incidences range from approximately 0.004 to 0.007%, with higher prevalence among young and middle-aged men. A total of 60–80% of affected patients reportedly develop unilateral ocular involvement only, while around 30–45% tend to have poor visual prognosis [95][147]. Intra-ocular infection is a dangerous complication in patients with SLE as a result of immunosuppression. The symptoms of autoimmune diseases are alleviated with methotrexate and azathioprine [96][148]. Glucocorticoids are contraindicated in central serous chorioretinopathy, however, the use of glucocorticoids in appropriate doses has been used in a patient with SLE associated with renal failure and rapid decrease in vision who had no history of glucocorticoids use, for alleviating both the systemic and ocular manifestation of SLE [95][147]. A good visual prognosis can be achieved by early diagnosis and aggressive treatment with intravenous cyclophosphamide [67][119]. Cyclophosphamide and Rituximab have been used aggressively, as reported by other authors, in managing ocular manifestations such as retinal vascular occlusion and occlusive retinal vasculitis [97][98][149,150]. Systemic steroids, hydroxychloroquine sulfate (HCQS), blood thinners, immunosuppressants, and laser photocoagulation have been used in managing the posterior complications of SLE [99][100][151,152]. An initial symptom of SLE may be optic nerve dysfunction but this usually occurs during the course of the disease. Optic neuritis and ischemic optic neuropathies are implications of the effect of SLE on the optic nerve which results in progressive vision loss and may lead to complete blindness [101][153]. Abnormal pupil responses, cranial nerve palsies, ocular motility defects, internuclear ophthalmoplegia, transient monocular vision loss, cortical visual impairment, and varying scotomas are less common presentations underlining neuropathies seen in SLE [102][154]. It should, however, be noted that multiple researcheuthors have opined that central visual field testing and microperimetry are not good diagnostic tests for SLE [103][155]. Optic neuritis in SLE is a result of an ischemic process that leads to subsequent demyelination. Patients’ best-corrected visual acuity correlates to the degree of axon loss. A total of 50% of patients with optic neuritis end up progressing to optic nerve atrophy. The early administration of corticosteroids (intravenous pulsatile steroid therapy), followed with continuously tapered oral doses, and addition of DMARDs, e.g., cyclophosphamide and methotrexate, can mitigate damage [3]. Table 1 summarizes some less common signs and symptoms reported in patients suffering SLE.Table 1.
Signs and symptoms reported in patients suffering Systemic Lupus Erythematosus (SLE).
| Authors | Signs and Symptoms |
|---|---|
| Chauhan et al. [104][156], Budoff and Tsui [105][157] | Fronsted branch angiitis. |
| Li et al. [106][158], Garcia-Soler et al. [107][159] | Purtscher-like retinopathy |
| Prakash et al. [108][160] | Simultaneous occlusion of the central retinal artery and vein, and optic neuritis |
| Subasi et al. [109][161] | Changes in macular and peripapillary microvascular density |
| David et al. [110][162] | Chorioretinopathy |
| Alhassan et al. [111][163], Kuthyar et al. [112][164], Wu et al. [113][165] | Retinal vasculitis |
| Zhang et al. [114][166] | Unilateral branched retinal occlusion |
| Liu et al. [115][167], Shi et al. [116][168] | Retinal thickness and microvascular alterations |
| Lin et al. [117][169], Wang et al. [118][170] | Scleritis, keratitis, and orbital cellulitis |
| Braga et al. [119][171], Agin et al. [120][172] | Choroidal thickness alterations |
| Fischer et al. [121][173] | Child-onset chorioretinopathy |
| Invernizzi et al. [122][174] | Drusenoid retinopathy in young adult |
| Kumar et al. [123][175] | SLE flare up resulting in retinopathy |
| Monov et al. [124][176] | Acute necrotizing scleritis |
| Park et al. [125][177] | Acquired enophthalmos. |
| Jeyachandran et al. [126][178] | Optic neuropathy |
| Hu and Peng [127][179] | Macular infarction |
| Chin et al. [128][180] | Polypoidal choroidal vasculopathy |
| Baglio et al. [129][181] | Choroidopathy |
| Salazar et al. [130][182] | Nine syndromes |
| Fraga et al. [131][183] | A cohort of pediatric SLE patients were reported to have significant chloroquine-induced retinopathies and other ocular morbidities |
