Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by si He and Version 2 by Catherine Yang.
As a biomedical material, porous titanium alloy has gained widespread recognition and application within the field of orthopedics. Its remarkable biocompatibility, bioactivity, and mechanical properties establish it as a promising material for facilitating bone regeneration. A well-designed porous structure can lower the material’s modulus while retaining ample strength, rendering it more akin to natural bone tissue. The repair and replacement of a wide range of bone defects caused by diseases, trauma, and aging has been an important subject for centuries.
- 3D printing
- titanium alloys
- bone implant
- porous structure
1. Porosity
Porosity is the ratio of the porous portion to the solid portion of the scaffold. Porosity characteristics are usually obtained indirectly through physical measurements (for example, using the density principle, immersing the sample in water, placing it in a graduated cylinder, replacing the volume of water with the actual volume of the scaffold, and obtaining porosity data based on the difference in volume) or by using digital image processing and analysis, as well as computed tomography techniques that can provide a more direct way to obtain porosity data [1][47]. The porosity is calculated as follows:

Here, Vp represents the pore volume of the scaffold, while Vz denotes the original volume of the scaffold.
The elastic modulus of implants is mainly regulated by porosity and can be changed by adjusting the porosity. At the same time, porosity plays an important role in establishing early bone integration and forming strong interface bonding between porous implants and surrounding tissues [2][48]. Increasing porosity provides more room for cells to grow, and the transport of oxygen and nutrients is correspondingly increased [3][49]. However, adding too high a porosity with the same structural design can lead to a significant decrease in mechanical properties, so finding the optimal porosity range is crucial for the successful application of the implant. The right porosity can provide cells with room to reproduce while mimicking the pore structure and mechanical strength of natural bone tissue [4][15]. When the porosity of the implant matches that of the human bone, the optimal bone growth environment can be obtained. Natural bone tissue comprises two distinct structures: cancellous bone and cortical bone. The interior of cancellous bone features a spongy structure with a porosity ranging from 50% to 90%. The internal structure of cortical bone is dense, the bone density is much lower than that of cancellous bone, and the porosity is only 5% to 10%. Scaffolds with a porosity comparable to that of human bone trabeculae (70% to 90%) have been shown to enhance cell viability and inward bone growth [5][6][50,51]. Moreover, studies have shown that when the porosity is greater than 70%, the porosity of the porous structure can have a beneficial effect on bone tissue [7][52]. A scaffold structure with a 600–900 μm pore size and 60%–90% porosity is recommended as the best structure [8][53]. Zhang et al. [9][54] differentiated the pore rate of a 3D-printed preparation (40%, 70%, and 90%), and the pore diameter was 700 μm multipores. Micro-CT results showed that the bone integration effect of the implant with a porosity of 40% (P40) was inferior to that of the implant with a porosity of 70% (P70) and 90% (P90). In addition, it is suggested that the change in pore size has a more significant effect on osteogenesis when the porosity is in the range of 70%–90%. In recent years, more and more researchers have prepared porous titanium alloys with a gradient structure. Hindy et al. [10][40] follow the biomimetic approach and apply the porosity gradient visible in natural bone to the fabrication of orthopedic and dental implants. The replication of this functional gradient ensures the correct distribution of the compression stiffness in different regions.
2. Pore Configuration
Most studies only explored the porosity and pore size suitable for endogenic bone growth under the condition of a fixed pore shape and ignored the fact that the pore shape of a porous structure would also affect the effective spatial distribution of the cells inside the scaffold and affect the mechanical properties of the scaffold. The pore shape was originally designed to mimic the shape of micropores inside the natural human bone, which is a complex tissue with a precise porous structure. There are different opinions about the micropore structure of bone tissue. Some people think that the microholes inside the human bones are round, some people observe that they are square holes, and some people think that they are hexagonal honeycomb holes. The geometry of the holes in bone implants can be square, rectangular, spherical, trabecular, or hexagonal, and more complex shapes can be made by using solid free-form fabrication techniques, such as cubes [11][57], diamonds [12][58], rhombohedrons [13][41], and variations of these structures. With different pore shapes, the mechanical properties and osteogenic properties of the scaffolds are also different. For instance, the diamond structure has two additional angles compared to the cubic structure, thereby offering a larger adhesive surface area for cells. The internal topologies of the porous materials designed by computer-aided methods can be roughly divided into (1) spatially arranged units composed of pillars, (2) three-period minimum surfaces (TPMSs), and (3) irregular bioinspired or Voronoi Mosaic structures. In recent years, TPMSs have also been widely applied to the field of bone tissue engineering based on naturally occurring nanoscale spiral structures found on butterfly wings that have an average curvature value of zero while the average curvature value of human trabecular bones is also close to zero [14][17]. A TPMS is an infinite and periodic surface, and the TPMS is often described by the following types of equations:
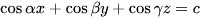
This equation satisfies the equation φ (x, y, z) = c, and this function φ (x, y, z) is the isosurface evaluated by the isosurface c.
TPMS structures, including gyro, primitive, and diamond structures, are generated by using mathematical formulas to tune their mechanical properties by changing various parameters such as the periodicity and relative density. Kelly et al. [14][17] evaluated the performance of TPMS titanium scaffolds produced by AM to repair femoral defects in rats and confirmed that TPMS scaffolds can repair segmental bone defects. Table 12 summarizes studies of the biological properties of AM implants with different structures. Jahir-Hussain et al. [15][59] conducted a comparative analysis of the mechanical properties of 3D-printed polylactide (PLA) scaffolds with four distinct pore structures, including round, square, hexagonal, and triangular, by utilizing finite element analysis (FEA). Their findings revealed that scaffolds featuring hexagonal pore shapes exhibited mechanical properties consistent with those of human bones. Van Bael et al. [16][60] discovered that, in comparison to hexagonal holes, triangular holes were more favorable for cell growth and differentiation, whereas rectangular holes were more prone to causing cell blockage. By examining local curvature and pore shapes, it was determined that obtuse angles were more likely to result in cell blockage compared to acute angles. However, Xu et al. [17][61] reported that the osteogenic ability of hexagonal prism scaffolds was higher than that of triangular prism scaffolds through in vivo and in vitro studies. Zhao et al. [18][62] reported the influence of tetrahedral and octahedral cell scaffolds on cell affinity and found that octahedral cells exhibit better static mechanical properties and a longer fatigue life than tetrahedral cells. At the same time, cells spread better on the scaffold on the octahedron than on the tetrahedron.
Kovács et al. [19][63] studied the mechanical properties and bone inward growth effect of titanium alloy scaffolds with six lattice shapes, including a gyro type, cube, cylinder, tetrahedron, diagonal cone, and Tyson polygon. The efficiency of the bone inward growth of several lattice shapes was compared, and the results showed that the bone growth degree of the gyro, conical, and cubic lattices was the best. Lim et al. [20][64] also came to the same conclusion by implanting titanium scaffolds of three different structures (octadense, gyroid, and dode) into the femur of rabbits, and no differences in bone formation in the titanium scaffolds were observed between the three types of pore structures. Farazin et al. [21][65] compared the biocompatibility of the cube, pyramid, and diagonal pore structures and found that the pyramid structures had the highest cell viability and migration ability. Deng et al. [22][66] conducted a study to investigate the effect of 3D-printed scaffolds with four different pore structures (i.e., diamond, tetrahedral cells, round pores, and cubes) on the osteogenic properties. The results showed that the diamond structure produced the best bone growth, possibly because the structure’s strut angles are similar to the angles between the trabeculae of cancellous bone in humans. At the same time, fluid dynamics (CFD) studies also show that the diamond structure has the smallest fluid velocity difference and the longest fluid flow path. This property is very beneficial for promoting blood vessel development, promoting nutrient transport, and enhancing bone formation. Therefore, the diamond structure is more conducive to bone growth. Compared with diamond structures, rhombohedral dodecahedrons have been shown to have better mechanical strength and moderate biological properties and can be applied to body parts with relatively high mechanical properties requirements [23][67]. Zhao et al. [4][15] conducted a study on the mechanical properties of supports featuring various pore structure elements. The findings revealed that supports with diamond-shaped pore elements exhibited the lowest compressive strength, measuring only approximately 38.2 MPa. Supports featuring cyclopore elements displayed a lower compressive strength, around 57.0 MPa, while those with cube-shaped pore elements demonstrated a higher compressive strength, approximately 142.8 MPa. In summary, the diamond structure, rhombohedral dodecahedron structure, and cube structure show great potential in promoting bone regeneration.
Table 12.
Common porous scaffold structures and their characteristics.
| Pore Size (μm) | Porosity (%) | Pore Structure | Cell Structure | Conclusions | Ref. |
|---|---|---|---|---|---|
| 500 | 80 75 70 65 60 |
Diamond Gyroid Orthogonal Cube Truss |
 |
Truss and cube structures have higher compressive strength. Diamond and gyroid structures have lower compressive strength, which may be due to the complex porosity and small vertical solid-bearing surface of these two structures. | [4][15] |
| 100 | — | Circular Square Hexagonal Triangular |
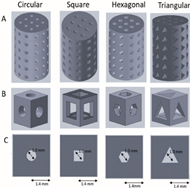 |
The results of the finite element analysis (FEA) indicate that scaffolds with a hexagonal pore shape exhibit greater similarity in performance to human bones. | [15][59] |
| 500 | — | Triangle Hexagon Rectangle |
 |
Rectangular pore is easy to cause cell blockage. Compared with hexagonal, triangular pore structure is more conducive to cell growth and differentiation. | [16][60] |
| 500 1000 |
67 62.87 84 77 |
Tetrahedron Octahedron |
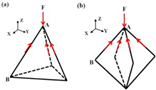 F represents compressive stress, and the red arrow represents tensile stress. |
The adhesion of scaffolds with 1000 apertures was superior, but their compressive and fatigue properties were inferior to those of scaffolds with 500 apertures. Octahedral scaffolds exhibited better compression performance and fatigue life compared to tetrahedral scaffolds, and they also displayed a greater capacity for cell proliferation. | [18][62] |
| 1076 739 |
70 | Gyroid | 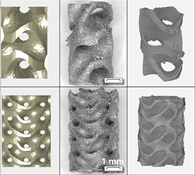 |
Titanium gyroid-sheet scaffolds can be used to repair segmental defects, and small-hole gyroscaffolds exhibit considerable inward-facing growth compared to large-hole gyroscaffolds. There was no significant difference in torsional strength and stiffness of the small pore implant compared with intact femur. | [14][17] |
| 600 | — | Gyroscope Cube cylinder tetrahedron Double pyramid Voronoi |
 |
Six lattice shapes, gyroscope, cube, cylinder, tetrahedron, double pyramid, and Voronoi, were ranked for bone growth efficiency, and gyro, double pyramid, and cube lattice implants had the highest bone tissue growth per unit time. | [19][63] |
| 1070 300 760 |
75 | Octedens Gyroid Dode |
 A: pore size, B: strut thickness. |
No differences in bone formation in titanium scaffolds were observed between the three types of pore structures. | [20][64] |
| 650 | 65 | Diamond Tetrahedro Cell Circular Cube |
 |
Diamond structure has the best bone growth, and fluid dynamic analysis also shows that this structure is conducive to blood vessel growth and bone formation. | [22][66] |
3. Pore Size
The porous structure not only efficiently lowers the elastic modulus, encouraging the formation of a mineralized layer on the implant’s surface and promoting protein adsorption, but also offers a conducive environment for cell adhesion, thereby facilitating the proliferation and differentiation of bone cells. Additionally, it serves as a channel for the transmission of metabolism and nutrition [24][68]. The pore size is very important for porous implants, and it affects the expression of osteogenic genes and the differentiation of osteoblasts. Wang et al. [25][69] confirmed that pore size and structure also play a certain role in regulating the expression of genes related to angiogenesis. The aperture of the scaffold should ensure that bone cells, nerve fibers, and blood vessels can grow into the scaffold. When the aperture is too large, it increases air permeability, which prevents cells from adhering to the surface [26][70]. When the aperture is too small, cells cannot enter the scaffold, resulting in cell accumulation, reducing cell migration in the scaffold and even affecting the circulation of nutrients and metabolic waste, which is not conducive to the growth of bone tissue [7][52]. At present, there is no precise definition of the most suitable pore size for bone growth. Some studies generally believe that the pore size of 100 μm to 400 μm can promote angiogenesis and bone growth, and below this range will limit bone cell growth [27][71]. Through a comprehensive analysis of the pore size required for the internal growth space of bone tissue and the formation of blood vessels, it is recommended that the optimal pore size is 300–600 μm [28][29][72,73]. The comprehensive impact of pore size on implants is summarized and drawn Figure 13. After staining with Toluidine Blue, it was observed that new bone tissue had developed within nearly all of the surface micropores of the 600 μm implants. The results indicate that, in comparison to scaffolds with 200 μm and 1000 μm apertures, the scaffolds featuring 600 μm apertures were more favorable for the growth of new bone tissue. Zhao et al. [18][62] reported tetrahedral cell titanium alloy scaffolds with pore sizes of 500 μm and 1000 μm.
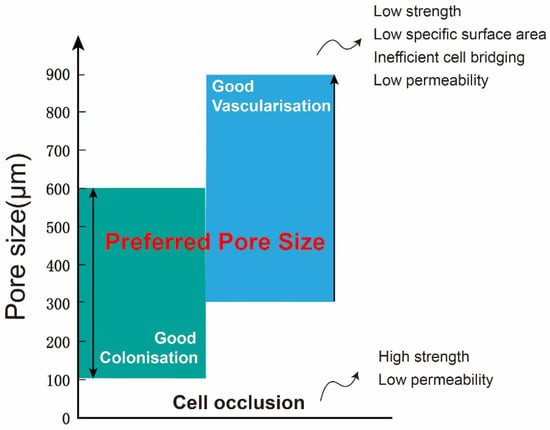
Figure 13.
Comprehensive effect of pore size on implants.
