1. Introduction
1.1. General Consideration on Lipid Metabolism
The second most prevalent hematologic cancer, multiple myeloma (MM), is characterized by the clonal proliferation of malignant plasma cells. The use of proteasome inhibitors, immunomodulatory medicines, anti-CD38 monoclonal antibodies, autologous transplants, and the B-cell maturation antigen targeting therapy has improved overall survival even though the condition is still mostly incurable. Regretfully, despite these developments, patients who continue to worsen following treatment have a low median survival rate
[1]. Because of this, identifying new players in the beginning and development of the pathology is crucial to identifying new targets for treatment.
The interaction of the marrow microenvironment with malignant plasma cells was a significant discovery
[2]. It is now widely recognized that the bone marrow microenvironment, which includes, among other cell types, bone marrow adipocytes, fibroblasts, stromal cells, osteoclasts, osteoblasts, and endothelial cells, influences the clinical course of MM, and each of these cell types plays a role in the pathogenesis and progression of the disease.
New data about this complicated environment points to a potential involvement for bone marrow adipocytes in the course of the disease. Dyslipidemia seems to be a newly identified prognostic marker for the course and fate of diseases, suggesting that the dysregulation of the lipoprotein transport system may be crucial to the appearance, prognosis, and even treatment of diseases. A number of studies have shown that changes in lipid metabolism and solid tumors are closely related.
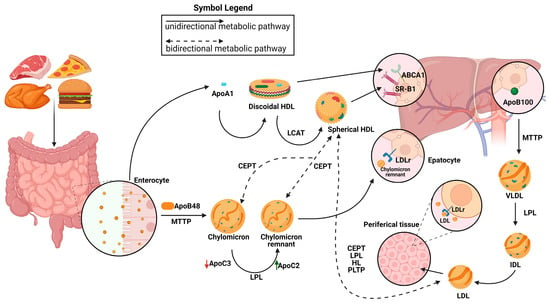
The biogenesis of high-density lipoproteins (HDLs) occurs exclusively in systemic circulation with the participation of apolipoproteins, lipid transporters, such as ATP binding cassette A1 (ABCA1) and G1 (ABCG1), and the plasma enzyme, such as lecithin–cholesterol acyltransferase (LCAT). HDL metabolism also involves additional steps, in which the plasma enzyme cholesteryl ester transfer protein (CETP) mediates the exchange of cholesteryl ester (CE), present in HDLs, for triglycerides (TGs) present in triglyceride-rich lipoproteins (TRLs) (chylomicrons, chylomicron remnants, and very low-density lipoproteins [VLDLs]). This enzymatic exchange is a critical crossing point for all three lipoprotein pathways, where TGs from TRLs enter the HDL pathway, and CE from HDLs enter the chylomicron and VLDL pathways
[3].
The chylomicron pathway, which is critical for the absorption and distribution of dietary lipids, and the VLDL/intermediate density lipoprotein (IDL)/low density lipoprotein (LDL) pathway, which is essential for the delivery of endogenously synthesized lipids from the liver to the peripheral tissues, and the HDL pathway, which is essential for the redistribution of peripheral cholesterol and other lipids among various tissues, are the metabolic pathways that are responsible for transporting lipids in circulation. Apolipoproteins, enzymes, lipid transfer proteins, and lipoprotein receptors are only a few of the many proteins that are involved in these pathways and support lipid homeostasis in general
[4].
After lipids are loaded onto Apolipoprotein B48 (Apo B-48) molecules by the intestinal microsomal triglyceride transfer protein (MTTP), chylomicrons are formed within the enterocytes. Chylomicrons are produced and then secreted into the bloodstream via lymphatic circulation. There, they undergo conversion to chylomicron remnants by lipoprotein lipase (LPL) and acquire apolipoprotein E (ApoE), which facilitates their removal by low-density lipoprotein receptor (LDLR) superfamily members. Hepatic MTTP facilitates the transfer of hepatic lipids onto Apolipoprotein B100 (Apo B100) during VLDL assembly, resulting in the production of nascent VLDL particles that are subsequently released straight into systemic circulation. Like chylomicrons, VLDL triglycerides (TGs) are digested by plasma LPL, first transforming into IDL and then into LDL particles, which are subsequently eliminated from the bloodstream by the LDL receptor superfamily’s members. LDL, VLDL, and chylomicron remnants are eliminated from the bloodstream mainly by means of LDL receptor superfamily members (LDLR and LRP1)
[5]. It has also been proposed that Heparan sulfate proteoglycans (HSPGs) are involved in this process, maybe by drawing lipoproteins that are in circulation onto cell membranes
[6][7][6,7] and exposing them to LDL receptors
[8], LRP1, VLDLR, and scavenger receptors. It has been demonstrated that HSPGs, primarily syndecan-1, functions as a separate receptor for TG-rich lipoprotein remains in the liver
[9]. Furthermore, ApoE is assumed to facilitate the interaction between LPL and HSPGs, which reinforces the LPL group’s stability
[10][11][10,11]. The ability of HSPGs to respond to important tumor microenvironmental stressors, such as hypoxia, by increasing the recruitment of HDL, LDL, and VLDL particles, is one of its new roles
[12] (
Figure 1).
Figure 1. Representation of metabolic pathways of lipoprotein. Chylomicrons are synthesized from the intestine and are elaborated by the members of the low-density lipoprotein (LDL) receptors. Very low-density lipoprotein (VLDL)/intermediate density lipoprotein (IDL)/LDL particles are produced by the liver and the LDL clearance is due to the LDH receptors. The high-density lipoprotein (HDL) metabolic pathway involves the synthesis of discoidal HDLs and the formation of mature spherical HDLs. Their clearance is due to the action of the SR-BI receptor. The exchange of lipids between HDLs, chylomicrons, chylomicron remnants, and VLDLs is also represented. LPLs are essential for the conversion from chylomicron (CM) to CM remnants in the exogenous pathway and from VLDLs to LDLs in the endogenous pathway. Apolipoprotein C-II (APOC2) is a critical factor for LPL activity. Apolipoprotein C-III (APOC3) competes with LPL for binding to lipid emulsion particles. MTTP: microsomal triglyceride transfer protein; CEPT: cholesteryl ester transfer protein; LPL: lipoprotein lipase; PLTP: phospholipid transfer protein; ABCA1: ATP-binding cassette transporter; ApoB100: apolipoprotein B; ApoB48: apolipoprotein B48; LCAT: lecithin–cholesterol acyltransferase.
1.2. HDL Metabolism and Cancer
The primary component of the cell membrane, lipids, are crucial for cell shape and proliferation
[13], and previous research has suggested that changes in lipid metabolism may be linked to specific types of carcinogenesis
[14], but there are contradicting findings in the literature.
Since the HDL is the lipoprotein that is most prevalent in most species, it is likely that this particle has significant roles. A rising body of research indicates that the HDL may have anti-inflammatory, anti-oxidative, and anti-apoptotic effects in addition to regulating innate and adaptive immune responses
[15][16][17][18][15,16,17,18]. Low HDL levels may contribute to the development of cancer by disrupting some of these characteristics
[18].
Variations in HDL cholesterol levels have been linked to an increased risk of many cancers, including colorectal, lung, and endometrial cancers
[19][20][21][22][23][24][25][26][27][28][29][30][19,20,21,22,23,24,25,26,27,28,29,30]. Nevertheless, sometimes the findings have been inconsistent. Furthermore, it is unclear which protein component of the HDL, apolipoprotein A1, best describes the connection between the HDL and cancer risk
[31].
However, according to a Mendelian randomization meta-analysis of the APOE gene, Asians had a 14% higher chance of developing cancer overall for every 1 mg/dL decrease in their genetically determined HDL-C level. According to prospective research, individuals in the highest HDL-C quintile among 27,074 male smokers in Finland had a 65% reduced risk of non-Hodgkin lymphoma (NHL) than those in the lowest quintile
[22]. It is interesting to note that during the first ten years of follow-up, this inverse correlation was strongly found, but not during the subsequent follow-up. On the other hand, there was no significant correlation found between the risk of cancer and changes in total cholesterol, LDL-C, or triglyceride levels
[20].
In another study, Knekt et al. demonstrated that men in the lowest quartile of total cholesterol had a five times higher risk of lymphoma or leukemia compared to those in the higher quintiles among non-smokers
[32]. Nonetheless, a Mendelian randomization analysis revealed that, in contrast to genetically lowered LDL-C levels, low plasma LDL-C levels were robustly related with an increased risk of cancer
[33]. These data may imply that low LDL-C levels do not cause cancer.
Lastly, a study that evaluated low plasma HDL cholesterol and apolipoprotein A1 as cancer risk factors examined participants from two population-based cohorts: the Copenhagen General Population Study (107,341 patients) and the Copenhagen City Heart Study (9387 patients) and followed them prospectively until the end of 2016
[34]. They saw 8748 malignancies in the Copenhagen General Population Study and 2164 in the Copenhagen City Heart Study throughout a 25-year follow-up period. Multivariable adjusted hazard ratios (HRs) for any cancer were 1.13 for people with HDL cholesterol levels of 58–77 mg/dL, 1.18 for people with HDL cholesterol levels of 39–58 mg/dL, and 1.29 for people with HDL cholesterol levels < 39 mg/dL in the Copenhagen General Population Study, when compared to people with HDL cholesterol levels ≥ 77 mg/dL. Accordingly, HRs for any cancer were 1.06 for those with apolipoprotein A1 levels of 160–189 mg/dL, 1.18 for those with apolipoprotein A1 levels of 130–159 mg/dL, and 1.28 for those with apolipoprotein A1 levels < 130 mg/dL, in contrast to those with apolipoprotein A1 levels > 190 mg/dL. Low levels of HDL cholesterol and/or apolipoprotein A1 were linked to a higher risk of breast cancer, lung cancer, nervous system cancer, non-Hodgkin lymphoma, and myeloproliferative neoplasm among twenty-seven different cancer types. The overall findings in the Copenhagen City Heart Study for men and women individually were comparable. Thus, there is a correlation between the elevated risk of many malignancies and HDL levels. The strongest increases in risk were seen in hematological and nervous system cancers, with smaller increases in breast and respiratory cancers.
1.3. HDL Metabolism and Hematological Malignancies
Data presented in the last experimentation
[34] allow
reus
earchers to conclude that an important role appears to exist in the field of hematological neoplasms as well. Indeed, a recent study
[35] verified the associations between HDL-C levels and the main categories of blood malignancies. Using data from the Korean National Health Insurance Service, a competing risks regression model was utilized to look at the hazard ratios of hematologic malignancies in 9,596,145 persons. The following hematologic tumors were measured for incidence: myeloid leukemia (ML), lymphoid leukemia (LL), NHL, and Hodgkin lymphoma (HL). In 79,179,225 persons who received follow-ups for 8.3 years on average, 15,864 incident hematologic malignancies were found. Subjects in the lowest HDL-C quartile had the highest risk of all hematologic cancers combined as well as of each specific kind of blood cancer, as comparison to those in the highest HDL-C quartile. A low HDL-C level was found to be linked to an elevated risk of hematologic malignancy, indicating that it is a preclinical marker and independent risk factor for hematologic malignancy. The authors have unequivocally shown that a low HDL-C level is linked to a higher risk of both general and specific forms of hematologic malignancies. This negative correlation held true for all study participant subgroups.
A further study that assessed the serial change in the lipid profile of 238 individuals who had hematologic tumors looked into a different facet of the relationship between lipids and hematological malignancies
[36]. The HDL-C level in this group was lower than in the control group. Furthermore, compared to the control group, the patient group’s HDL-C level difference was more pronounced across all other lipid fractions. Interestingly, when patients experienced remission following treatment, the HDL-C level was reversed. The risk of NHL was found to be strongly correlated with the HDL-C level, but not with the total cholesterol or non-HDL-C level, according to the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study
[22]. HDL-C is the most sensitive measure of tumor burden, according to research conducted by Moschovi et al. on children with acute lymphoblastic leukemia
[37]. After reaching remission, the abnormally low HDL-C values at the time of the leukemia diagnosis were restored. Alford et al. looked into the connection between lymphomagenesis and serial changes in cholesterol levels. They concluded that cholesterol levels drop years before a diagnosis of cancer
[38].
2. HDL and Multiple Myeloma
2.1. HDL Effects in Multiple Myeloma
It is unclear how exactly MM patients’ low lipid levels are caused to manifest, and it has yet to be determined if changes in metabolism and the beginning of gammopathy are causally related.
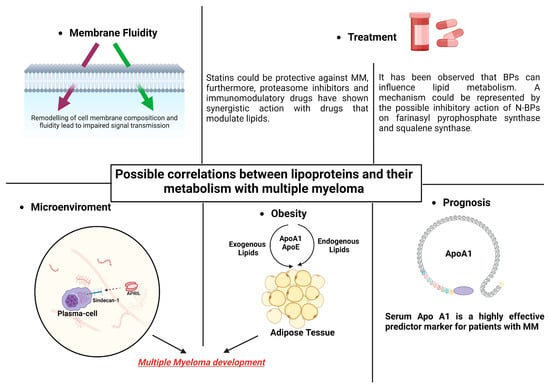
TAs we shall see in the next paragraphs, the effects of HDLs in myeloma disease probably occur at various levels. Some of the ways that HDLs work include through their impacts on cellular fluidity, their impact on the medullary microenvironment, and their effects on obesity and concomitant dyslipidemia. The association between dyslipidemia and hematological pathology is confirmed by the relationship shown between anti-dyslipidemic medications and MM and between anti-myeloma therapies and HDLs, as will be shown later (
Figure 2).
Figure 2. Lipid modifications that can alter the fluidity of MM cells’ membranes may be the cause of alterations in intracellular signaling. The development of MM and obesity may be influenced by lipoprotein’s actions on adipocytes. Combining statins and bisphosphonates with MM therapy may have synergistic effects. In individuals with MM, apolipoprotein might be significant in terms of prognosis.
First of all, cholesterol may be used by malignant cells. In general, cholesterol is crucial for the formation and function of cell membranes in both normal and malignant cells
[39][105], and the structure and functionality of the cell membrane may be a potential mechanism that, at least partially, mediates the effects of the lipid and lipoprotein transport system in the development of MM.
The extracellular stimulants of cell division and proliferation primarily target the cell membrane and its protein constituents. Fluidity is a crucial physical characteristic of cell membranes and is essential to their biological activity
[40][106]. Changes in the cholesterol content of the cell membrane have been shown to have an effect on membrane fluidity
[41][107], which can then have an effect on the structure, antigenicity, and responsiveness of proteins on the cell surface, such as G-coupled receptors and ion channels, to external stimuli. For instance, a reduced erythrocyte plasma membrane lipid content is associated with an increased activity of erythrocyte Na
+/Li
+ countertransport, Na
+/K
+ cotransport, and Na
+/K
+ pump activity
[42][108]. Consequently, this could have an impact on biological reactions and intracellular signaling, like in the instance of pancreatic B-islets secreting insulin. The physical characteristics of the membrane can be altered by lipoproteins, which can also assist in regulating the activity of transmembrane proteins and the motor-dependent elongation of interior organelles, including the endoplasmic reticulum
[43][44][45][46][109,110,111,112].
Several studies have established the existence of basic connections between inflammation and MM oxidative stress
[47][48][49][50][113,114,115,116]. It has also been discovered that the HDL, by its anti-inflammatory and antioxidative qualities, regulates inflammatory pathways in the progression of cancer
[51][117]. The HDL and its ApoA may cause immune cells’ lipid rafts and free cholesterol levels to drop, which would in turn lessen signaling pathways that promote cell proliferation and inflammation
[52]. ApoA’s antitumorigenic properties were well illustrated in an in vivo investigation. Through indirect changes to macrophage and other immune cell function, the mechanism by which this happens may have anticancer consequences
[53][47]. Furthermore, via HDL-related pathways, SR-BI is a key player in the control of the quiescence, differentiation, and proliferation of hematopoietic stem and progenitor cells. Moreover, compared to other cell types, the expression of SR-BI is noticeably higher on the surfaces of tumor cells. HDL cholesteryl esters can be taken up by SR-BI and absorbed into the cytoplasm, which lowers plasma HDL levels significantly
[54][55][44,54]. Therefore, a premalignant state may potentially be the cause of the low HDL level.
Elevated levels of oxidized LDL cholesterol are correlated with a low concentration of HDL cholesterol. Consequently, increased oxidative stress levels inside cells have been connected to the pathophysiology of cancer
[56][57][118,119]. Therefore, a low quantity of LDLs may result in less ox-LDLs and less damage to long-lived plasma cells from oxidation.
Recent in vitro research, however, suggests that exogenous cholesterol is a necessary metabolite for the survival of myeloma cells. However, the enhanced absorption of LDLs by myeloma cells may account for the low LDL plasma levels seen in MM patients
[58][120]. On the other hand, low levels of HDL, LDL, and total cholesterol may be the cause of cancer growth.
Like in solid tumors, the relationship between lipoproteins and obesity may also have a value in the case of MM. There is mounting evidence that the lipid and lipoprotein transport systems, which regulate endogenously created and exogenously obtained lipids through diet, are key players in the development of morbid obesity. Diet-induced obesity is also largely influenced by a number of other factors, including the most prevalent protein component of HDLs, APOA1, and ApoE, a protein component of HDLs, LDLs, and VLDLs, and the functional ligand of LDLR in the removal TRLs from circulation
[59][60][61][62][63][64][65][66][121,122,123,124,125,126,127,128]. Thus, it is conceivable that APOA1 and ApoE have an impact on the mechanisms linked to the onset and advancement of MM.
Furthermore, syndecan-1 has been demonstrated to be a receptor for TG-rich lipoprotein remnants in the liver, as previously described. MM cells have a surface protein called syndecan-1, which is essential for their interaction with the bone marrow microenvironment
[67][68][69][70][71][129,130,131,132,133]. High levels of cell surface syndecan-1 expression are a hallmark of myeloma tumors, with the HS chains of this protein being crucial for MM cell proliferation and survival in the BM microenvironment
[72][134]. Additionally, it has been discovered that syndecan-1 functions as APRIL’s co-receptor
[73][135]. APRIL is a crucial component of the BM microenvironment and promotes the survival of MM cells. Notably, myeloma growth in vivo is stimulated by the soluble form of syndecan-1
[74][136]. Furthermore, MM cell proliferation was inhibited, and the rate of apoptosis increased when syndecan-1 was suppressed
[75][137].
2.2. Prognostic Value of HDL in Multiple Myeloma Patients
In MM, HDLs can potentially serve as a stand-in prognostic marker
[76][138]. In Zhongshan Hospital in Shanghai, China, 307 MM patients were enrolled retrospectively between 2007 and 2016
[77][139]. The pre-diagnostic serum lipid profile’s predictive importance was assessed by the authors. Using the prognostic factors that were found, a new Lasso Cox regression model was built. The results demonstrated a substantial change in lipid levels between the ISS stages: the late ISS stage had reduced levels of LDL, cholesterol, and Apo A1 and B. But in terms of cause-specific survival (CSS), progression-free survival (PFS), and overall survival (OS), only Apo A1 demonstrated statistical relevance. Longer OS was seen in those with greater Apo A1 levels. Moreover, a multivariate analysis showed that Apo A1 was an independent predictor. Compared to the Durie and Salmon (DS) system and the International Staging System (ISS), the Zhongshan Score model demonstrated superior accuracy. In conclusion, serum Apo A1 is a highly effective predictive marker for patients with MM out of all the serum lipid profiles. The prognosis of MM patients, lipid content and ratios, and treatment responses are correlated, which implies that lipid profiles may have clinical implications for judgment and decision-making.
Lastly, a study sought to investigate a predictive risk-stratification model combining lipid profiles and clinical characteristics in patients with multiple myeloma
[78][140]. 275 patients’ data were retrospectively analyzed, and the training and validation cohorts were split up at random. A univariate and multivariate Cox analysis was used to identify the prognostic markers, which included HDL, TG, LDL, Apo B, and Apo A1 ratios, and lactate dehydrogenase. The construction of a six-factor prognostic model was based on a Lasso regression model. Following patient classification into low- and high-risk groups, the former group exhibited a longer OS duration. The risk score model’s area under the curve (AUC) for the five- and ten-year OS was 0.756 and 0.940, respectively, showing higher accuracy than the DS stage and the ISS.
To create a prognostic model, this study integrated the lipid metabolic profile with the clinical features of individuals with MM. The prediction accuracy was improved by the nomogram that integrated the risk score and ISS stage. This model can monitor the lipid profile as an easy-to-use and practical tool, which has some clinical importance for enhancing prognostic accuracy and identifying targets for treatment.