You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by José Pérez de la Lastra and Version 2 by Catherine Yang.
Polyphenols are involved in enzyme regulation, with a wide range of biological activities, and can interact with proteins through hydrophobic interactions, hydrogen bonding, and electrostatic interactions. These interactions can influence the function of enzymes, potentially altering their activity.
- antioxidants
- diseases
- vitamins
- polyphenols
- human diet
1. NADPH Oxidase
Elevated levels of ROS are constitutive in cancer. They are an important hallmark derived from increased production in mitochondria and by the NADPH oxidase (NOX, nicotinamide adenine dinucleotide phosphate oxidase) family of enzymes [1][228]. NOX is a membrane-bound enzyme complex that faces the extracellular space, and it can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. NADPH oxidase catalyzes the production of an •O2− by transferring one electron to O2 from NADPH [2][14]. The overall reaction for the formation of •O2− from NADPH is as follows (Figure 15):
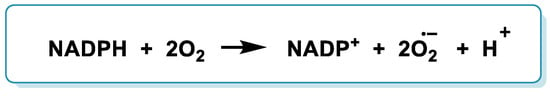
Figure 15.
Reaction for the formation of
•
O
2−
from NADPH.
If NOX expression is not properly regulated, NOX-associated ROS can promote OS, aberrant signaling, and genomic instability [3][229]. NOX isoforms are already known to be overexpressed in multiple malignancies, making them potential therapeutic targets in cancer. If NOX expression is not properly regulated, NOX-associated ROS can promote OS, aberrant signaling, and genomic instability [3][229]. NOX isoforms are already known to be overexpressed in multiple malignancies, making them potential therapeutic targets in cancer [4][230].
Several studies have investigated the potential of polyphenols to inhibit NOX activity and reduce ROS production, and it has been observed that these natural compounds prevent NOX expression [5][90]. Several compounds that have been studied are resveratrol [6][231], quercetin [7][232], EGCG [8][233], and curcumin [9][234]. The possible mechanism by which polyphenols inhibit NOX are by blocking the assembly of the NOX complex, necessary for the enzyme activity [10][235], and acting in the NOX electron transport chain [11][236].
2. Cyclooxygenase 2
COX-2, also known as cyclooxygenase 2 or prostaglandin-endoperoxide synthase 2, is an enzyme that plays a key role in the biosynthesis of prostanoids, which include prostaglandins, prostacyclins, and thromboxanes. This enzyme is inducible, meaning that it is not normally detected in most tissues, but its production can increase in response to certain stimuli, such as inflammation [12][237]. However, in some structures such as the ovary, prostate, kidney, and central nervous system, COX-2 may have a structural character. It is important to mention that, although COX-2 has traditionally been seen as an enzyme that is expressed only under pathological conditions, it has detrimental effects on the pathophysiology of diseases such as Alzheimer’s disease [13][238]. In relation to COX-2, polyphenols may have several effects: (i) Some studies have suggested that polyphenols may inhibit COX-2 activity [14][239], and (ii) wine polyphenols have been shown to exert an antineoplastic effect on the androgen-resistant PC-3 cell line through inhibition of NF-κβ-mediated transcriptional activity of the COX-2 promoter. This could explain, at least in part, the induction of apoptosis in vitro by these substances in castration-resistant prostate cancer [15][240].
3. Lysyl Oxidase
Lysyl oxidase (LOX) plays an important role in extracellular matrix (ECM) stabilization and may be related to endothelial dysfunction induced by atherosclerotic risk factors [16][241]. Inhibition of LOX may impair endothelial barrier function. In addition, it has been proposed that it has roles in atherogenesis and endothelial dysfunction, ocular disorders, fibrosis, iatrogenic diseases, bone regeneration, and increased risk of cardiovascular diseases, among others [17][242]. LOX catalyzes the oxidative deamination of lysine and hydroxylysine residues in collagen and elastin, two major structural proteins found in the ECM. This reaction produces aldehydes, which then form covalent cross-links between collagen and elastin molecules, strengthening the ECM and providing resistance to mechanical forces [18][243]. Polyphenols can influence the activity of LOX in different ways. Some polyphenols, such as chlorogenic acid, gallic acid, and caffeic acid, have been shown to have amine oxidase-like activity, which means that they can mimic the action of LOX and participate in collagen cross-linking. This suggests that polyphenols may contribute to the strengthening of the ECM and the maintenance of tissue integrity [19][244].
4. Lipoxygenase
Lipoxygenases (LOs) are a family of enzymes that catalyze the addition of oxygen to polyunsaturated fatty acids (PUFAs), specifically those containing a 1,4-pentadiene structure [20][245]. This reaction results in the formation of hydroperoxides, which can then be further metabolized into a variety of bioactive molecules, including leukotrienes, hydroxyeicosatetraenoic acids (HETEs), and fatty acid epoxides [21][246]. Los are found in a wide range of organisms, including plants, animals, and fungi, and dysregulation of LO activity can have significant implications in various diseases [22][247]. Polyphenols can affect the activity of enzymes such as LO, modulating the inflammatory process [23][72]. This is thought to be one of the mechanisms by which polyphenols can help to reduce inflammation and protect against chronic diseases [24][248].
5. Xanthine Oxidase
Xanthine oxidase (XO) is a form of xanthine oxidoreductase, a type of enzyme that generates ROS. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid [25][249]. They play an important role in the catabolism of purines in some species, including humans. The following chemical reactions are catalyzed by XO (Figure 216):
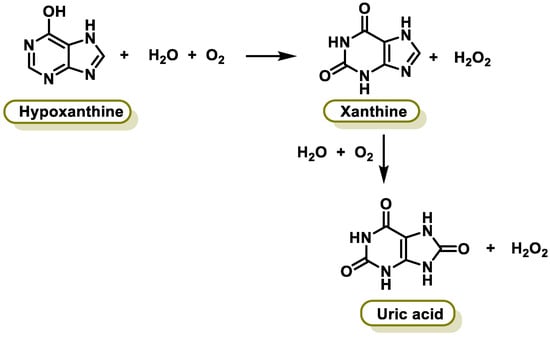
Figure 216.
Chemical reactions catalyzed by xanthine oxidase.
Inhibition of xanthine oxidase reduces the production of uric acid, and several medications that inhibit xanthine oxidase are indicated for treatment of hyperuricemia and related medical conditions. Polyphenols, particularly flavonoids, are known to have antioxidant properties and can act as potent inhibitors of XO activity [26][250]. XO is the main contributor of FR during exercise, but it is also involved in the pathogenesis of several diseases such as vascular disorders, cancer, and gout [27][251]. Several studies have indicated that the capacity of flavanols and flavones to inhibit the active site is largely dependent on hydrogen bonds between the polyphenol ligand hydroxyl groups and the catalytic residues of the binding site [28][252].
6. α-Synuclein
α-synuclein (αS) is a neuronal protein that is abundantly expressed in the brain, specifically in presynaptic nerve endings, constituting more than 1% of the total protein in the cytosol of brain cells. It is the major component of Lewy bodies in both sporadic and inherited forms of Parkinson’s disease and in Lewy body dementia. αS is a key protein in the pathology of Parkinson’s disease (PD) [29][253], characterized by the loss of dopaminergic neuronal cells in the substantia nigra pars compacta and the abnormal accumulation and aggregation of αS in the form of Lewy bodies and Lewy neurites. αS is the main component of Lewy bodies and is a pathogenic feature of all synucleinopathies, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). All of these diseases are determined by the deposition of αS aggregates, but can be separated into distinct pathological phenotypes and diagnostic criteria [30][254]. Kenjiro Ono et al., 2020, studied the impact of the polyphenolic acids 3-hydroxybenzoic acid (3-HBA), 3,4-dihydroxybenzoic acid (3,4-diHBA), and 3-hydroxyphenylacetic acid (3-HPPA) (derived from gut microbiota-based metabolism of dietary polyphenols) on the brain, and demonstrated their ability to inhibit αS oligomerization and mediate aggregate αS-induced neurotoxicity in vitro [31][255].
7. Receptor Tyrosine Kinases
Receptor tyrosine kinases (RTKs) are transmembrane proteins that act as signal transducers. They regulate essential cellular processes such as proliferation, apoptosis, differentiation, and metabolism. RTKs play an important role in cancer progression and are activated in response to environmental signals by initiating appropriate signaling cascades in tumor cells [32][256]. Alteration of RTKs occurs in a broad spectrum of cancers, emphasizing their crucial role in cancer progression and as a suitable therapeutic target [32][256]. It has been demonstrated that EGCG, a type of polyphenol, can lower levels of EGFR, a type of RTK, by both inhibiting transcription of the encoding gene and inducing internalization followed by degradation [33][257]. Another study identified tyrosine kinase inhibitors from Panax bipinnatifidus and Panax pseudoginseng, which are rich in polyphenols [34][258].
8. Histone Deacetylases
Histone deacetylases (HDACs) are a class of enzymes that remove acetyl groups from lysine residues on histone proteins. These modifications play a crucial role in regulating gene expression by altering the chromatin structure. The removal of acetyl groups by HDACs leads to the condensation of chromatin, making the DNA more tightly wound around histone proteins [35][259]. This compact chromatin structure hinders the access of transcription factors and RNA polymerases, consequently repressing gene expression. Conversely, histone acetylation by histone acetyltransferases (HATs) loosens chromatin, facilitating gene transcription [36][260]. HDACs are involved in a wide range of biological processes, including cell growth, differentiation, apoptosis, and metabolism. Dysregulation of HDAC activity has been implicated in several diseases, such as cancer, neurodegenerative disorders, and metabolic disorders [37][261]. HDAC inhibitors are a class of drugs that selectively inhibit HDAC activity. These inhibitors are being investigated as potential therapeutic agents for various diseases, including cancer, Alzheimer’s disease, and type 2 diabetes [38][262]. By inhibiting HDAC activity, these drugs aim to reverse the repressive effects of HDACs on gene expression and restore normal cellular function. Some flavonoids have been reported to act as HDAC inhibitors [39][263]. Choi et al., 2016, reported that piceatannol (a resveratrol metabolite found in red wine) affected HDAC expression in a mouse model and concluded that it may be a valuable therapeutic agent in renal fibrosis by decreasing HDAC4 and HDAC5 protein expression [40][264].
9. α-Amylase and α-Glucosidase
α-amylase and α-glucosidase are two important enzymes involved in carbohydrate digestion. They play crucial roles in breaking down starch, the main carbohydrate found in grains, legumes, and vegetables, into smaller sugar molecules that can be absorbed by the body. α-amylase is found in both salivary glands and the pancreas. It acts on starch by breaking down α-1,4-glycosidic bonds, which are the bonds that connect glucose units together. This process produces smaller chains of glucose molecules called dextrins and maltose. α-glucosidase is also found in the pancreas and small intestine. It further breaks down dextrins and maltose into glucose, the simplest form of sugar. This allows glucose to enter the bloodstream and be used for energy [41][265].
Inhibition of these two enzymes can be a useful strategy for controlling blood sugar levels in people with diabetes. This is because it can slow down the digestion of carbohydrates, which helps to prevent rapid spikes in blood sugar after eating. There are a number of different ways to inhibit α-amylase and α-glucosidase, including dietary strategies, natural inhibitors, and pharmaceutical agents (these agents are typically used to treat type 2 diabetes) [42][266].
There are several reports indicating the anti-diabetic capabilities of polyphenols through the inhibition of carbohydrate-hydrolyzing enzymes [43][267]. Flavonoids are explored as inhibitors of α-amylase, whereas polyphenols are thought to regulate starch digestibility [44][268]. According to Lo Piparo et al., 2008, the efficacy of inhibition is usually correlated with the amount of OH on the B-ring of the flavonoid [45][269].
