You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Takuji Tanaka and Version 2 by Catherine Yang.
Warthin’s tumor is the second most frequent neoplasm next to pleomorphic adenoma in the salivary gland, mostly in the parotid gland. The epithelial cells constituting a tumor are characterized by the presence of mitochondria that undergo structural and functional changes, resulting in the development of oncocytes.
- Warthin’s tumor
- pathogenesis
- neoplastic
1. Introduction
Warthin’s tumor, also known as papillary cystadenoma lymphomatosum, monomorphic adenoma, or adenolymphoma, is the second most common tumor of the parotid gland after pleomorphic adenoma, accounting for approximately 15% of all parotid tumors, and is encountered relatively frequently in daily clinical practice [1][2][1,2]. An investigation by Franzen et al. [3] suggested that Warthin’s tumor was the most common histological type in the period from 1997 to 2017. The researchers also suggested a growing incidence in women and a decreasing age of patients [3]. The site of occurrence is restricted to the parotid gland and surrounding lymph nodes, with a high frequency of simultaneous or ectopic multiple or bilateral occurrence [4][5][4,5]. The tumor usually presents as a painless mass, but it may be painful when the lesion is associated with inflammation [6]. Clinically, an ultrasound examination reveals the tumor as an oval and well-defined mass with multiple anechoic areas, or an anechoic mass with posterior acoustic enhancement. Rapid growth is stimulated by infection. In some cases, multiple septa and intra-tumoral fluid thickness can cause non-uniform echo patterns [7][8][9][7,8,9]. Treatment is usually based on surgical resection; however, most patients have low malignancy rates. Therefore, conservative treatment may be an option if Warthin’s tumor is preoperatively diagnosed [10][11][10,11].
2. Histopathology of Warthin’s Tumor
The histopathology of Warthin’s tumor is defined by the tubular, cystic, and papillary proliferation of highly cylindrical oncocyte-like cells with eosinophilic granular sporulation. It is well demarcated from the surrounding normal salivary gland tissue [12][13][13,14]. The tumor is also characterized by a biphasic arrangement of similar oncocyte-like cuboidal cells with cylindrical cells on the basal side. The stroma is occupied by mature, non-atypical small lymphocytes with lymph follicles (germinal centers), although the number of stroma varies from case to case [2] (Figure 12a). This may be caused by an immune response to the tumor epithelium or by residual lymphoid tissue within the lymph nodes that is partially replaced by the tumor epithelium [14][15]. In addition, the cytoplasm of cells exhibiting oncocytes possesses an excessive accumulation of mitochondria [15][16]. This may be a result of the accumulation of senescent mitochondria due to the reduction in cellular mitophages and is associated with a deletion of 4977 bp in the mitochondrial genome [16][17][18][19][17,18,19,20]. It is not uncommon for intermingled goblet cell-type mucous cells, glandular hairy metaplastic cells, and squamous metaplastic cells to be observed. Basal cells do not usually differentiate into myoepithelial cells. The cystic structure, lined with epithelial cells, is filled with necrotic contents, including cholesterol crystals. When the cystic structures rupture, the fluid leaks into the lymphangitic stroma, causing epithelioid granulomas with neutrophil infiltration. In association with this, squamous epithelium and mucous cells sometimes appear due to inflammatory or chemogenic changes, and in rare cases, extensive necrosis may accompany the lesions. Neither the epithelial nor the lymphocytic component is usually atypical; however, secondary adenocarcinoma, mucoepidermoid carcinoma, squamous cell carcinoma, oncocytic carcinoma, or malignant lymphoma (follicular lymphoma) may occur [20][21][22][23][24][25][26][27][21,22,23,24,25,26,27,28]. The most common histologic type of carcinoma derived from Warthin’s tumor is squamous cell carcinoma, although mucoepidermoid carcinoma has also been reported [28][29][29,30].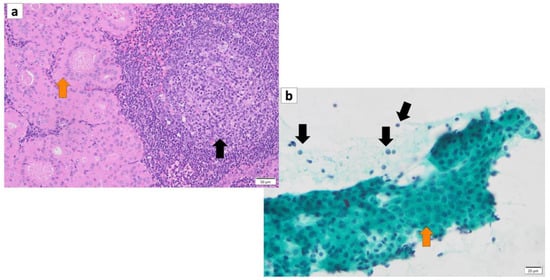
Figure 12. (a) Histopathology of Warthin’s tumor. Note: varying proportions of papillary cystic structures are lined with bilayered oncocytic epithelial cells (orange arrow) and surrounded by a lymphoid stroma, including germinal centers (black arrow). (b) FNA cytology of Warthin’s tumor shows small cohesive sheets of oncocytes with abundant granular cytoplasm with a central round nucleus/prominent nucleolus (orange arrow). Lymphocytes (black arrows) with granular debris in the background. (a) Hematoxylin and eosin staining (Bar represents 50 μm) and (b) Papanicolaou staining (bar represents 20 μm).
3. Cytology of Warthin’s Tumor
A cytological diagnosis of Warthin’s tumor can be made by observing a two-cell pattern of lymphocytes and epithelial cells, with lymphocytes in the background and eosinophilic cells in clusters or sporadically isolated. The aggregates of oncocytes range in size from large to small sheets without abnormal overlapping [30][31]. On Papanicolaou staining smears, oncocytes appear with light green stained granular cytoplasm, often with eosinophilic changes that stain orange G. Their nuclei are small and slightly atypical, sometimes with a few small nucleoli, but they are often obscure. Occasional findings include cystic structures, including oncocytes floating in the lumen (Figure 12b). When cyst contents of punctured cells contain hypercylindrical nucleated oncocyte cells along with histiocytes, the diagnosis of Warthin’s tumor is inferred; however, in the absence of nucleated oncocyte cells, the diagnosis is more challenging [31][32]. In May–Giemsa-stained specimens, Warthin’s tumor cells do not possess a metachromatic component, and in some cases, there are various mature lymphocytes in the background. The cellular presentation of squamous metaplastic Warthin’s tumor, which is a secondary alteration of Warthin’s tumor, is a mixed appearance of neutrophils and histiocytes with orange G-stained metaplastic squamous cells [32][33]. In addition, mucous cells often appear with metaplasticity, in which case mucous cells are interspersed with collections of oncocytes on a mucous background. For the diagnosis of Warthin’s tumor, fine needle aspiration cytology (FNAC) is useful in the preoperative diagnosis of salivary gland tumors because it is minimally invasive and has many typical cytological findings [33][34][35][34,35,36]. Data have been reported that FNAC has a sensitivity of 93%, a specificity of 94.8%, and an accuracy of 94.6% in the diagnosis of Warthin’s tumor [35][36].4. Risk Factors for Warthin’s Tumor
Warthin’s tumors were first reported over 100 years ago, but their pathogenesis is not fully understood [36][12]. Various possible pathogeneses and risk factors have been described. (1) The majority of Warthin’s tumors show an obvious marginal sinus beneath the tumor capsule and have an intralymphatic origin or a metaplasia of normal salivary gland epithelial or ductal cell origin [37][38][39][40][37,38,39,40]. (2) Catalytically inactive glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was found to bind to damaged mitochondria and incorporate these mitochondria directly into lysosomes, exhibiting a characteristic immunohistochemical GAPDH staining pattern in Warthin’s tumor cells, suggesting either whole cell progressive loss of cytoplasmic GAPDH (Figure 23), likely due to loss or nuclear shift of the protein [17][18]. (3) The epithelium of Warthin’s tumor, whether hyperplastic, metaplastic, or neoplastic, interacts with lymphoid tissue [2]. (4) A high association with smoking, which causes chronic inflammation of the epithelium, has been reported [41][42][43][41,42,43]. (5) The incidence of Warthin’s tumor is reported to be high after radiation exposure [44]. (6) Patients with Warthin’s tumor have been reported to show an increased incidence of autoimmune or infectious diseases [36][45][12,45]. (7) It has been reported that angiogenesis and lymphangiogenesis are increased, reactive lymphocyte hyperplasia is induced, and that the two elements, epithelial cells and lymphocytes, are not simply present by chance but are interdependently related to tumor development [46]. (8) HPV infection is reportedly associated with the development of Warthin’s tumor [47]. (9) IgG4-reated disease (IgG4-RD) has been reported to be indirectly involved in the development of Warthin’s tumor [48]. To date, there is no consensus on the development of Warthin’s tumor.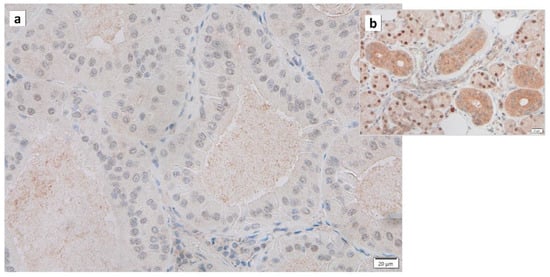
Figure 23. Immunohistochemistry of GAPDH in Warthin’s tumor. Note the (a) negative reaction in the columnar epithelial cells and (b) positive reaction in the cytoplasm of intercalated ductal cells and some nuclei of acinar cells. Bars represent 20 μm.
