You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sergey Alferov.
PubMed (NCBI) has pointed to an exponential growth of publications on the subject of a “biofuel cell” in the first decade of our century, and this interest persisted throughout the following years. It should be noted that biofuel elements based on microorganisms (microbial fuel cells, MFCs) are a promising technology to produce bioelectricity since they simultaneously solve the problems of contamination with anthropogenic organic waste, which can be used by microorganisms as a source of carbon and energy.
- plant microbial fuel cell
- electrogenic microorganisms
- biofuel cells
1. Introduction
The ubiquitous environmental pollution due to various anthropogenic substances, such as heavy metals [1], petroleum products [2], medicinal preparations [3], and pesticides [4], is one of the main problems of mankind nowadays. Pollutants have a negative impact not only on the environment but also on human life, accumulating in heterotrophic food chains and entering the human body, which leads to various diseases of the nervous system and respiratory organs, as well as genetic abnormalities while reducing life expectancy [1]. The above-described problems are reflected in the UN Sustainable Development Goals; according to the developed programs of the United Nations Environment Programme (UNEP) and UN-Water, the control over the global pollution of ecosystems and their restoration are of high priority [5].
Bioremediation, a complex of purification methods using the metabolic potential of biological objects, is applied to purify soil and water ecosystems from pollutants. Thus, the introduction of microorganisms into ecosystems makes it possible to dispose of various pollutants by converting them to simpler safe substances. The principle of phytoremediation is based on the binding and accumulation of pollutants in plant vacuoles [6]; it activates a complex metabolic pathway involving the antioxidant plant protection system [7]. Additionally, plants and microorganisms (fungi and bacteria) interact with each other at the root level (in the rhizosphere), showing a positive synergistic effect in the elimination of pollutants such as heavy metals and organic compounds [8,9][8][9]. The disadvantages of the bioremediation of soils include the low rate of toxicant biodegradation, as well as the need for a thorough preliminary examination of the contaminated site to clarify the modes of biotechnological work. This requires high labor and energy costs, such as the plowing and irrigation of fields and the disposal of waste plants. Therefore, this technology is not widely used in developing countries and is unattractive in poor countries [10].
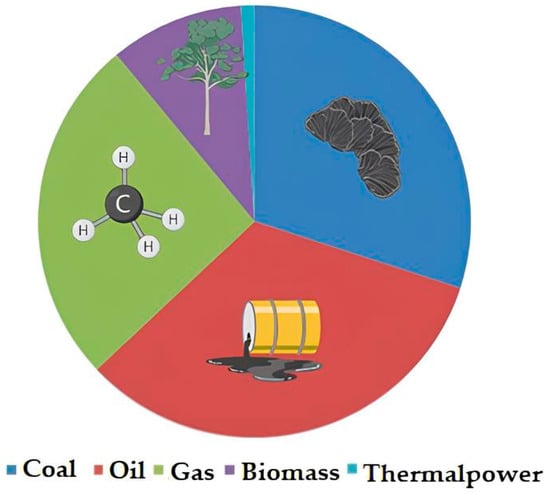
A significant contribution to atmospheric pollution is made by heat and power stations (hereinafter referred to as CHP) operating on traditional fuel sources (coal, oil, and gas); their share (Figure 1) is about 60% of the global electricity generation [11]. The issue of using renewable energy sources (RES) for electricity generation is relevant, considering the trend programs of many developed countries towards the reduction of carbon dioxide emissions into the atmosphere and providing access to inexpensive, reliable, sustainable, and modern energy for all segments of the population [12]. These include solar panels, wind generators, and biofuels [13].
However, renewable energy sources have a number of disadvantages. Thus, the process of their disposal is an extremely difficult task [15] since, for example, solar panels contain elements such as As, Cd, Hg, and Pb [16], which can have a negative impact on the ecosystem, and their burial is an extremely undesirable method of their disposal [17]; thermal and chemical methods of solar panel recycling have not been sufficiently mastered and are not characterized by a high degree of efficiency [18,19][18][19]. Dust is released during the mechanical processing of solar panels. It contains glass fiber, noise pollution is created, and rare earth elements are lost. However, 80 million tons of waste from used solar panels are expected worldwide by 2050, which will inevitably have a negative impact on the surrounding ecosystem.
The use of biofuel cells (BFCs) is an effective alternative in this context, as electricity generation is carried out in the process of the biocatalytic oxidation of various substrates. Despite the low power generated in the BFC system and, as a result, a long payback period, the research in the field of biofuel elements is relevant due to humanity’s awareness of global environmental problems, the need to solve which reduces the role of economic levers in the development of the world community. PubMed (NCBI) has pointed to an exponential growth of publications on the subject of a “biofuel cell” in the first decade of our century, and this interest persisted throughout the following years. It should be noted that biofuel elements based on microorganisms (microbial fuel cells, MFCs) are a promising technology to produce bioelectricity since they simultaneously solve the problems of contamination with anthropogenic organic waste, which can be used by microorganisms as a source of carbon and energy. A continuous and steady supply of organic substrates is required to ensure the uninterrupted generation of electricity in an MFC, which cannot always be implemented in practice. A fairly new technology of plant microbial fuel cells (hereinafter referred to as PMFCs) eliminates this disadvantage of MFCs largely. The electricity generation is carried out via the oxidation of organic substances using microorganisms that are both synthesized in plants during photosynthesis under the action of sunlight energy and produced into the environment (root exudates, root deposits, and rhizo-deposition) and come from outside, for example, from wastewater or industrial waste. Such hybrid energy technology can be used in phytomonitoring the state of plant crops, a local power supply, charging portable devices [20], powering various low-power sensors to monitor ambient temperatures and humidity, power camera traps in remote areas [21], and serve as a biosensor for monitoring plant health in smart greenhouses [22] (Figure 2). It should be noted that the PMFC technology, using macrophytes, reduces the level of greenhouse gases (N2O and CH4) by 5.9–32.4% in terms of CO2 [23].
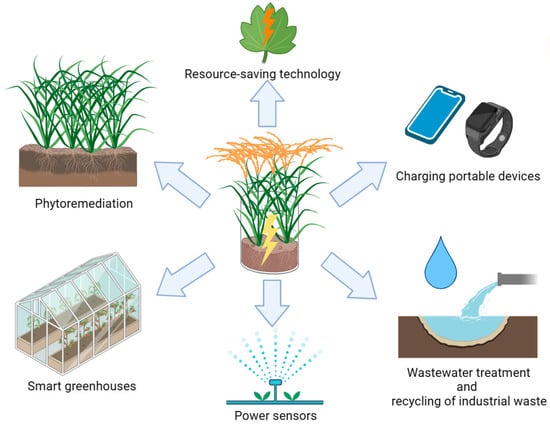
Figure 2.
Possibilities of PMFC application.
2. Plant Microbial Fuel Cells: Functioning and Factors Affecting the Electrochemical Characteristics of the SYSTEM
The generation of electricity depends on many factors, such as the types of exoelectrogenic microorganisms used, the material of the electrodes and their modification, environmental factors, and the plants used. Understanding the functioning principles and the optimal choice of microorganisms and plants makes it possible to increase the efficiency of electricity generation in a PMFC.
2.1. The Principle of PMFC Operation
The principle of PMFC operation is based on two interrelated processes: the synthesis of rhizo-deposits in plants and their use as a substrate by microorganisms to generate electricity (Figure 3). Complex interactions in heterogeneous, polydisperse, multifactorial natural systems were previously described as a computer model of the chemical and microbiological production processes of plant biomass, soil microorganisms, and nutrients in the rhizosphere [26][24].
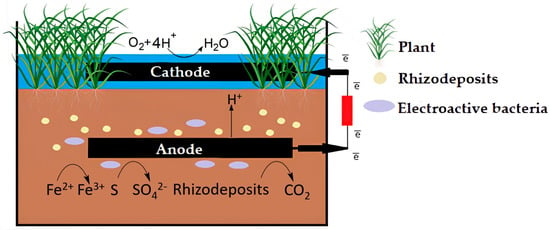
Figure 3.
The scheme of PMFC functioning.
Photosynthesis regulates the vital activity of plants, during which plants fix carbon dioxide from the atmosphere and form carbohydrates, organic acids and amino acids, secrets—polysaccharide mucus (mucigel), lysates—materials of dead cells, gases—ethylene ethylene and carbon dioxide under the influence of sunlight energy [27][25]. Electrogenic microorganisms use deposits as substrates for growth and development, as well as electricity generation as a result of ongoing oxidative processes involving the enzymatic systems of microorganisms. As a result, carbon dioxide is synthesized, and free charge carriers (protons and electrons) are formed. Charges need to be separated to convert chemical energy into electrical energy. The process is carried out by moving the generated electrons at the anode to the cathode through an external circuit; protons migrate through a nutrient matrix or medium from the substrate to the cathode due to the presence of a potential gradient [18], where molecular oxygen or another catalyst and water molecules are formed [28][26]. However, it is likely that hydroperoxyl radicals (HO2) are formed on the cathode during the reduction process as an intermediate product [29][27]. Microorganisms, in turn, can enter symbiosis with plant roots, forming protective biofilms and producing antibiotics to protect plants from pathogens [30][28].
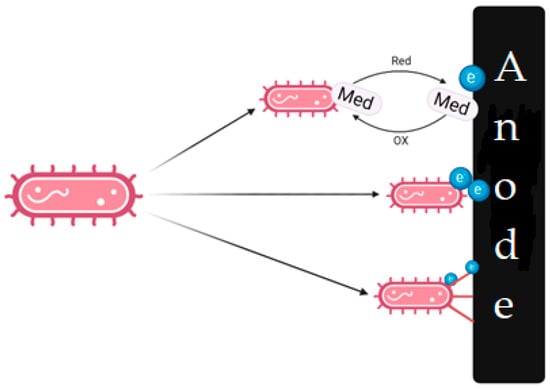
When choosing microorganisms, it is necessary to consider their ability to transfer electrons to the anode (Figure 4), which can be caused by various mechanisms: direct electron transfer through cytochromes and electron-conducting molecular saws (nanowires) with the help of electroactive compounds (mediator transfer). General information about this various mechanisms is summarized in recent reviews and articles [31,32,33,34,35,36,37][29][30][31][32][33][34][35].

Figure 4.

Various ways in which electron transfer to the anode can occur.
The focus is on natural ecosystems when choosing microorganisms for a PMFC system. Relatedly, bacteria inhabit the environment in the rhizosphere; they are anaerobes that produce protons and carbon dioxide and can transfer electrons to the anode during the oxidation of organic compounds. Table 1 presents a description of some rhizospheric bacteria.
Table 1.
Rhizospheric microorganisms capable of direct extracellular electron transfer.

| Microorganism | Description | Consumable Substrates | References |
|---|---|---|---|
| Desulfobulbus sp. | Obligate anaerobes capable of oxidizing sulfur to sulfate using an anode as an electron acceptor. | Acetate, propionate, butyrate, lactate, and pyruvate | [36][37][38] |
| Geobacter sp. | Anaerobic metal-reducing bacteria. Fe (III) and Mn (IV) are used as electron acceptors. They can transmit electrons using pili—filamentous protein formations. | Benzoate, p-cresol, trichloroethane, benzene, lactate, acetate, and starch | [39] |
| Geothrix fermentans | Anaerobic metal reducers. Fe (III) is used as an electron acceptor. They are capable of forming extracellular mediators of the quinone series and riboflavin, which makes it possible to transfer electrons to the electrode more efficiently. | Acetate, propionate, lactate, and fumarate | [40][41] |
| Rhodoferax ferrireducens | Facultative metal-reducing anaerobe with a wide temperature range of growth. Fe (III), Mn (IV), nitrate, fumarate, and oxygen can be used as electron acceptors. | Acetate, lactate, propionate, pyruvate, malate, succinate, and benzoate | [42] |
| Shewanella sp. | Facultative anaerobic bacteria using Fe (III) and Mp (IV) as electron acceptors are capable of producing flavins that act as electronic transfer mediators. | Lactate and formate | [43][44] |
| Clostridium butyricum C. beijerinckii |
Obligate anaerobes can use an anode as an electron acceptor. Hydrogen, which is able to oxidize at the anode, is produced during the enzymatic fermentation of substrates. | Glucose, starch, sucrose, and lactate | [45] |
The basic property of microorganisms that allows their use in bioelectric systems [48,49,50][46][47][48] is their ability to produce electroactive compounds, as well as to use an anode as an electron acceptor. Moreover, the use of inorganic anions as an electron acceptor makes it possible to reduce the salinity of treated wastewater [51[49][50],52], for example, when using sulfate-reducing bacteria that are capable of the assimilatory reduction of sulfates to sulfides [53][51].
PGPR (plant-growth-promoting rhizobacteria), which promote plant growth, play an important role in maintaining the vital activity of plants and are used for the development of PMFC. Such microorganisms include, for example, bacteria of the species Bacillus thuringiensis, which are involved in nitrogen fixation processes, sulfur and phosphorus exchanges, and the synthesis of plant growth stimulants [54][52]. Bacteria of the genus Pseudomonas sp. can be also considered as a PGPR-group bacteria [55][53]. Some species of Pseudomonas sp. are capable of surfactant destruction [56,57][54][55]; they can form biofilms on the surface of an anode and secrete compounds of the phenase-new series [58][56]. These compounds play an important role both in protecting plants from pathogen infection [59][57] and stimulating the growth of shoots [60][58]. Moreover, phenazines act as mediators of the electronic transport between bacteria and an electrode [61][59]. Bacteria of the family Ruminococcaceae spp. are not electroactive but are capable of utilizing cellulose (35–50% of the dry plant weight) while producing organic substrates, which are additionally used by electroactive microorganisms as electron donors [62][60]. Therefore, the use of PGPR-group bacteria can be used in PMFC systems to stimulate plant growth and protection, which theoretically can have a beneficial effect on electricity generation.
2.2. Electrodes in PMFC
It is important to choose the right electrode material for the efficient generation of electrical energy when creating PMFCs along with biological components [63][61]. Generally, the electrode material should have high electrical conductivity, electrochemical stability, porosity, and biocompatibility [64][62]. Metals (zinc [65][63], stainless steel [66][64], and platinum [67][65]) and carbon materials [68][66] are usually used as electrodes in bioelectrochemical systems. Despite the high electrical conductivity of metals in comparison with carbon materials, the use of stainless steel, for example, increases the period of microorganism adaptation on the metal anode surface [68][66]. It causes a decrease in current generation at the initial stage of the PMFC operation, which is explained by the lower biocompatibility of stainless steel to microorganisms. Moreover, metals are subject to corrosion processes [66][64] and have a high cost, thus limiting their use in PMFC development.
The geometric area of the electrodes affects the output of electricity—the larger the area, the more contact there is for electroactive microorganisms, which leads to an increase in current density [69][67]. In turn, graphite electrodes (felt/fiber) have a developed surface that promotes the adhesion of microorganisms and the sorption of organic compounds. This material is not subject to corrosion; therefore, it is promising for the creation of PMFCs [70][68]. The addition of granular graphite or activated carbon to the surface of the anode improves the adsorption of organic compounds and increases the specific surface area for colonization via bacteria. Electrode modification is used to improve the producible power of bioelectrochemical systems, which is described in detail in recent articles [71,72,73,74,75][69][70][71][72][73]. The use of carbon materials produced from crop waste is also promising in this field [76][74].
Thus, the choice of electrode material is the key element determining the efficiency of the entire PMFC system. Existing materials can be modified to reduce their internal resistance in order to increase the current output and power.
2.3. Application of Proton Exchange Membranes in PMFC System
Various PMFC configurations have been developed so far: sediment PMFCs, constructed-wetland MFCs, tubular PMFCs, floating-treatment wetland MFCs, flat plate PMFCs, and power-generating trees. The advantages and disadvantages of each model are detailed in the review [24][75]. One of the components of bioelectrochemical systems for power generation is a proton exchange membrane, which allows the improvement of charge segregation and power performance [77][76]. The most preferred proton exchange membrane is Nafion, but its use in BES significantly (by 40%) increases the cost of the device [78][77]. Thus, the search for new membranes that will have a lower cost and provide high stability and efficiency in BES is currently underway.
In [79][78], modified Nafion 117 proton exchange membranes were tested. The modification included the treatment of the membrane with solutions of polyvinylidene difluoride (PVDF) and sulfonated PVDF with the addition of silicon oxide (SiO2). The third modification involved the polymerization of a Nafion membrane in a methyl methacrylate (MMA) solution with the addition of sodium sulfite as an initiator. According to the results obtained, all three methods increase the power generation parameters of MFC systems. The highest increase in current density, from 0.81 mA/m2 to 18.82 mA/m2, was demonstrated using the modification of Nafion with MMA.
In [80][79], a proton exchange membrane based on agar and polyvinyl alcohol (PVA) with the addition of vermiculite nanoparticles was tested. According to the results obtained, the proton exchange properties of the tested membranes were 216% higher than those of the commercial Nafion membrane. In addition, the MFC current density increased (from 605 mA/m2 to 1515 mA/m2) when agar and PVA-based membranes were used. A low cost and environmental safety, in combination with the increased efficiency of MFC energy generation, allow the use of agar and PVA-based membranes as an alternative to expensive Nafion membranes.
Ceramic membranes based on clay, bentonite, coal ash, Na2CO3, Na2SiO3, and H3BO3 were considered in [81][80]. The use of hybrid ceramic membranes with the addition of different compounds contributed to the increase in PMFC power density by 78% (up to 22.38 mW/m2) compared to the control (100% clay membrane). There was a decrease in internal resistance from 346 Ω (control) to 234 Ω. The addition of bentonite, coal ash, Na2CO3, Na2SiO3, and H3BO3 improved the membrane’s cation transport, reducing oxygen diffusion to the anode chamber. The membrane demonstrated high stability during 6 months of PMFC operation. In addition, the ceramic membrane is significantly cheaper than the Nafion membrane.
Thus, one of the important aspects of PMFC operation, power increase, and internal resistance reduction is the use of proton exchange membranes. At the same time, for the commercialization of PMFC systems, it is necessary to take into account the cost of the production of such membranes and the expenses associated with the complication of the design when using membranes.
2.4. The Influence of Environmental Factors on the Electricity Generation in a PMFC
The metabolic activity of exoelectrogenic microorganisms, which play an important role in BES functioning and electricity generation, depends on the temperature, the pH, and the rate of organic substrates’ receipt. Thus, the work [82][81] showed that, when the air temperature rises to 30 °C, the voltage of the bioelectrochemical system increases from 100 to 150 mV, which may be due to an increase in the metabolic rate of exoelectrogenic microorganisms. The pH value affects the development of microorganisms. pH of 6–9 is mostly suitable for the functioning of BES [83][82]. The power decreases to 158 mW/m2 at a pH value of 6.0 for the MFC system [84][83], while the power value is 600 mW/m2 at a pH of 8.0. The inhibition of the metabolic activity of exoelectrogenic microorganisms is observed with a decrease in pH, which contributes to a decrease in the BES power [85][84].
Periodic watering is necessary for the normal functioning of plants since soil moisture affects the generated potential in a PMFC system. The article [86][85] states that, in the absence of irrigation, the soil dries up, which leads to a two-fold decrease in the PMFC potential, but after watering (60–70% of the soil moisture capacity), the potential is restored. Thus, energy generation changes depending on the time of day [87][86]. An increase in electrogenic activity is observed after sunrise due to the launch of photosynthesis processes, the peak of which is observed from 14 to 15 h. Depending on the system under study, the open circuit potential is 600–700 mV at the specified time. Then, the photosynthetic activity of plants decreases at nightfall, which leads to a decrease in electricity generation to 300–400 mV.
The rate of photosynthesis is affected by the concentration of carbon dioxide in the atmosphere [88][87]. The trend towards carbon dioxide emissions increases every year and is 390 ppmv, according to the latest data (mass fractions of a percent per volume), which is 30% more than the CO2 concentration in the early twentieth century [89][88]. The increasing CO2 concentration and climate warming significantly affect plant growth [90][89]. The work [91][90], using agricultural plants (Saccharum officinarum and Sorghum bicolor), showed that the rate of photosynthesis grows significantly with an increase in the CO2 concentration, which in theory can have a positive effect on the power produced via a PMFC. It should be noted that plants with the C3 and C4 types of photosynthesis react differently to an increase in the carbon–acid gas concentration. C4 plants attach CO2 to phosphoenolpyruvate [86][85], resulting in the formation of oxalic acid containing four carbon atoms. The photosynthesis efficiency of C4 plants is significantly higher since the C4 pathway is an extra pump that supplies additional portions of CO2, increasing its concentration in the plant since the CO2 concentration in the assimilation chamber is lower than in the air, which is a limiting factor of photosynthesis.
References
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589.
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526.
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal Variation in the Occurrence of Pharmaceuticals in Effluents from a Sewage Treatment Plant and in the Recipient Water. Environ. Sci. Technol. 2005, 39, 8220–8226.
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and Distribution of Pesticide Residues in Soil: Non-Dietary Human Health Risk Assessment. Chemosphere 2020, 253, 126594.
- UNEP Harnessing Opportunity: Wastewater as a Managed Resource. 2017; 2. Group, T.I.-D.W.; Programme, U.N.E. Freshwater Strategic Priorities 2022–2025. Available online: https://www.unep.org/resources/publication/harnessing-opportunity-wastewater-managed-resource (accessed on 24 September 2023).
- Praveen, A.; Pandey, V.C. Pteridophytes in Phytoremediation. Environ. Geochem. Health 2020, 42, 2399–2411.
- Tanwir, K.; Amna; Javed, M.T.; Shahid, M.; Akram, M.S.; Ali, Q. Antioxidant Defense Systems in Bioremediation of Organic Pollutants. In Handbook of Bioremediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 505–521. ISBN 978-0-12-819382-2.
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of Soils Polluted with Crude Petroleum Oil Using Bassia Scoparia and Its Associated Rhizosphere Microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120.
- Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. IJERPH 2021, 18, 2435.
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation Strategies for Soils Contaminated with Heavy Metals: Modifications and Future Perspectives. Chemosphere 2017, 171, 710–721.
- Shalaeva, D.S.; Kukartseva, O.I.; Tynchenko, V.S.; Kukartsev, V.V.; Aponasenko, S.V.; Stepanova, E.V. Analysis of the Development of Global Energy Production and Consumption by Fuel Type in Various Regions of the World. IOP Conf. Ser. Mater. Sci. Eng. 2020, 952, 012025.
- UNEP Harnessing Opportunity: Ensure Access to Affordable, Reliable, Sustainable and Modern Energy for All, 2017. Available online: https://www.un.org/en/chronicle/article/goal-7-ensure-access-affordable-reliable-sustainable-and-modern-energy-all (accessed on 4 September 2023).
- Song, F.; Mehedi, H.; Liang, C.; Meng, J.; Chen, Z.; Shi, F. Review of Transition Paths for Coal-Fired Power Plants. Glob. Energy Interconnect. 2021, 4, 354–370.
- EnerData: Global Energy & CO2 Data. Available online: https://www.enerdata.net/research/energy-market-data-co2-emissions-database.html (accessed on 20 September 2022).
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An Overview of Solar Photovoltaic Panels’ End-of-Life Material Recycling. Energy Strategy Rev. 2020, 27, 100431.
- Vezhenkova, I.; Semenova, M.; Kovalevskaya, A.; Gryaznov, A.; Rodríguez-Barroso, M.R.; Jimenez Castañeda, R. Chemical Composition Determination of Impurities and Effect on the Toxicity Degree of Solar Panel Components. E3S Web Conf. 2020, 220, 01057.
- Korniejenko, K.; Kozub, B.; Bąk, A.; Balamurugan, P.; Uthayakumar, M.; Furtos, G. Tackling the Circular Economy Challenges—Composites Recycling: Used Tyres, Wind Turbine Blades, and Solar Panels. J. Compos. Sci. 2021, 5, 243.
- Bandaru, S.H.; Becerra, V.; Khanna, S.; Radulovic, J.; Hutchinson, D.; Khusainov, R. A Review of Photovoltaic Thermal (PVT) Technology for Residential Applications: Performance Indicators, Progress, and Opportunities. Energies 2021, 14, 3853.
- Riech, I.; Castro-Montalvo, C.; Wittersheim, L.; Giácoman-Vallejos, G.; González-Sánchez, A.; Gamboa-Loira, C.; Acosta, M.; Méndez-Gamboa, J. Experimental Methodology for the Separation Materials in the Recycling Process of Silicon Photovoltaic Panels. Materials 2021, 14, 581.
- Prasad, J.; Tripathi, R.K. Scale-up and Control the Voltage of Sediment Microbial Fuel Cell for Charging a Cell Phone. Biosens. Bioelectron. 2021, 172, 112767.
- Osorio de la Rosa, E.; Vázquez Castillo, J.; Carmona Campos, M.; Barbosa Pool, G.; Becerra Nuñez, G.; Castillo Atoche, A.; Ortegón Aguilar, J. Plant Microbial Fuel Cells–Based Energy Harvester System for Self-Powered IoT Applications. Sensors 2019, 19, 1378.
- Brunelli, D.; Tosato, P.; Rossi, M. Flora Health Wireless Monitoring with Plant-Microbial Fuel Cell. Procedia Eng. 2016, 168, 1646–1650.
- Wang, X.; Tian, Y.; Liu, H.; Zhao, X.; Peng, S. The Influence of Incorporating Microbial Fuel Cells on Greenhouse Gas Emissions from Constructed Wetlands. Sci. Total Environ. 2019, 656, 270–279.
- Meshalkin, V.P.; Chetyrbotskii, V.A.; Chetyrbotskii, A.N.; Pelii, A.F. Computer Modeling of Chemical-Microbiological Processes in Rhizomicrobiophytospheric System. Dokl. Phys. Chem. 2020, 495, 171–175.
- Escolà Casas, M.; Matamoros, V. Linking Plant-Root Exudate Changes to Micropollutant Exposure in Aquatic Plants (Lemna Minor and Salvinia Natans). A Prospective Metabolomic Study. Chemosphere 2022, 287, 132056.
- Kabutey, F.T.; Zhao, Q.; Wei, L.; Ding, J.; Antwi, P.; Quashie, F.K.; Wang, W. An Overview of Plant Microbial Fuel Cells (PMFCs): Configurations and Applications. Renew. Sustain. Energy Rev. 2019, 110, 402–414.
- Khilari, S.; Pandit, S.; Das, D.; Pradhan, D. Manganese Cobaltite/Polypyrrole Nanocomposite-Based Air-Cathode for Sustainable Power Generation in the Single-Chambered Microbial Fuel Cells. Biosens. Bioelectron. 2014, 54, 534–540.
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192.
- Raghavulu, S.V.; Babu, P.S.; Goud, R.K.; Subhash, G.V.; Srikanth, S.; Mohan, S.V. Bioaugmentation of an Electrochemically Active Strain to Enhance the Electron Discharge of Mixed Culture: Process Evaluation through Electro-Kinetic Analysis. RSC Adv. 2012, 2, 677–688.
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical Transport along Bacterial Nanowires from Shewanella Oneidensis MR-1. Proc. Natl. Acad. Sci. USA 2010, 107, 18127–18131.
- Schröder, U. Anodic Electron Transfer Mechanisms in Microbial Fuel Cells and Their Energy Efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629.
- Reguera, G. Microbial Nanowires and Electroactive Biofilms. FEMS Microbiol. Ecol. 2018, 94, fiy086.
- Chong, G.W.; Karbelkar, A.A.; El-Naggar, M.Y. Nature’s Conductors: What Can Microbial Multi-Heme Cytochromes Teach Us about Electron Transport and Biological Energy Conversion? Curr. Opin. Chem. Biol. 2018, 47, 7–17.
- Liu, X.; Shi, L.; Gu, J.-D. Microbial Electrocatalysis: Redox Mediators Responsible for Extracellular Electron Transfer. Biotechnol. Adv. 2018, 36, 1815–1827.
- Lovley, D.R. Microbial Fuel Cells: Novel Microbial Physiologies and Engineering Approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332.
- De Schamphelaire, L.; Cabezas, A.; Marzorati, M.; Friedrich, M.W.; Boon, N.; Verstraete, W. Microbial Community Analysis of Anodes from Sediment Microbial Fuel Cells Powered by Rhizodeposits of Living Rice Plants. Appl. Env. Microbiol. 2010, 76, 2002–2008.
- Nosek, D.; Cydzik-Kwiatkowska, A. Microbial Structure and Energy Generation in Microbial Fuel Cells Powered with Waste Anaerobic Digestate. Energies 2020, 13, 4712.
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 59, pp. 1–100. ISBN 978-0-12-387661-4.
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix Fermentans Gen. Nov., Sp. Nov., a Novel Fe(III)-Reducing Bacterium from a Hydrocarbon-Contaminated Aquifer. Int. J. Syst. Evol. Microbiol. 1999, 49, 1615–1622.
- Mehta-Kolte, M.G.; Bond, D.R. Geothrix Fermentans Secretes Two Different Redox-Active Compounds to Utilize Electron Acceptors across a Wide Range of Redox Potentials. Appl. Env. Microbiol. 2012, 78, 6987–6995.
- Finneran, K.T. Rhodoferax ferrireducens Sp. Nov., a Psychrotolerant, Facultatively Anaerobic Bacterium That Oxidizes Acetate with the Reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 2003, 53, 669–673.
- von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of Flavins by Shewanella Species and Their Role in Extracellular Electron Transfer. Appl. Env. Microbiol. 2008, 74, 615–623.
- Wu, D.; Xing, D.; Mei, X.; Liu, B.; Guo, C.; Ren, N. Electricity Generation by Shewanella Sp. HN-41 in Microbial Fuel Cells. Int. J. Hydrog. Energy 2013, 38, 15568–15573.
- Niessen, J.; Schroder, U.; Scholz, F. Exploiting Complex Carbohydrates for Microbial Electricity Generation? A Bacterial Fuel Cell Operating on Starch. Electrochem. Commun. 2004, 6, 955–958.
- Das, S.; Calay, R.K. Experimental Study of Power Generation and COD Removal Efficiency by Air Cathode Microbial Fuel Cell Using Shewanella Baltica 20. Energies 2022, 15, 4152.
- Liu, S.; Feng, Y.; Li, H. Retracted: Effect of Geobacter Metallireducens Nanowire on Electron Transfer Efficiency in Microbial Fuel Cell. J. Chem. Tech. Biotech. 2023, 98, 473–482.
- Liu, Z.D.; Du, Z.W.; Lian, J.; Zhu, X.Y.; Li, S.H.; Li, H.R. Improving Energy Accumulation of Microbial Fuel Cells by Metabolism Regulation Using Rhodoferax ferrireducens as Biocatalyst. Lett. Appl. Microbiol. 2007, 44, 393–398.
- Hemalatha, M.; Shanthi Sravan, J.; Venkata Mohan, S. Self-Induced Bioelectro-Potential Influence on Sulfate Removal and Desalination in Microbial Fuel Cell. Bioresour. Technol. 2020, 309, 123326.
- Sun, M.; Tong, Z.-H.; Sheng, G.-P.; Chen, Y.-Z.; Zhang, F.; Mu, Z.-X.; Wang, H.-L.; Zeng, R.J.; Liu, X.-W.; Yu, H.-Q.; et al. Microbial Communities Involved in Electricity Generation from Sulfide Oxidation in a Microbial Fuel Cell. Biosens. Bioelectron. 2010, 26, 470–476.
- Miran, W.; Jang, J.; Nawaz, M.; Shahzad, A.; Jeong, S.E.; Jeon, C.O.; Lee, D.S. Mixed Sulfate-Reducing Bacteria-Enriched Microbial Fuel Cells for the Treatment of Wastewater Containing Copper. Chemosphere 2017, 189, 134–142.
- Treesubsuntorn, C.; Chaiworn, W.; Surareungchai, W.; Thiravetyan, P. Increasing of Electricity Production from Echinodosus Cordifolius-Microbial Fuel Cell by Inoculating Bacillus Thuringiensis. Sci. Total Environ. 2019, 686, 538–545.
- Anokhina, T.O.; Siunova, T.B.; Sizova, O.I.; Zakharchenko, N.S.; Kochetkov, V.V. Rhizospheric Bacteria of the Genus Pseudomonas in Modern Agrobiotechnology. Agric. Chem. 2018, 10, 54–66. (In Russian)
- Icgen, B.; Salik, S.B.; Goksu, L.; Ulusoy, H.; Yilmaz, F. Higher Alkyl Sulfatase Activity Required by Microbial Inhabitants to Remove Anionic Surfactants in the Contaminated Surface Waters. Water Sci. Technol. 2017, 76, 2357–2366.
- Chaturvedi, V.; Kumar, A. Diversity of Culturable Sodium Dodecyl Sulfate (SDS) Degrading Bacteria Isolated from Detergent Contaminated Ponds Situated in Varanasi City, India. Int. Biodeterior. Biodegrad. 2011, 65, 961–971.
- Arulmani, S.R.B.; Gnanamuthu, H.L.; Kandasamy, S.; Govindarajan, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A.; Zhang, H. Sustainable Bioelectricity Production from Amaranthus viridis and Triticum aestivum Mediated Plant Microbial Fuel Cells with Efficient Electrogenic Bacteria Selections. Process Biochem. 2021, 107, 27–37.
- Raio, A.; Reveglia, P.; Puopolo, G.; Cimmino, A.; Danti, R.; Evidente, A. Involvement of Phenazine-1-Carboxylic Acid in the Interaction between Pseudomonas chlororaphis Subsp. Aureofaciens Strain M71 and Seiridium cardinale In Vivo. Microbiol. Res. 2017, 199, 49–56.
- Yuan, P.; Pan, H.; Boak, E.N.; Pierson, L.S.; Pierson, E.A. Phenazine-Producing Rhizobacteria Promote Plant Growth and Reduce Redox and Osmotic Stress in Wheat Seedlings Under Saline Conditions. Front. Plant Sci. 2020, 11, 575314.
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408.
- Timmers, R.A.; Rothballer, M.; Strik, D.P.B.T.B.; Engel, M.; Schulz, S.; Schloter, M.; Hartmann, A.; Hamelers, B.; Buisman, C. Microbial Community Structure Elucidates Performance of Glyceria Maxima Plant Microbial Fuel Cell. Appl. Microbiol. Biotechnol. 2012, 94, 537–548.
- Chiranjeevi, P.; Yeruva, D.K.; Kumar, A.K.; Mohan, S.V.; Varjani, S. Plant-Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 549–564. ISBN 978-0-444-64052-9.
- Guo, K.; Donose, B.C.; Soeriyadi, A.H.; Prévoteau, A.; Patil, S.A.; Freguia, S.; Gooding, J.J.; Rabaey, K. Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environ. Sci. Technol. 2014, 48, 7151–7156.
- Ayala-Ruiz, D.; Castillo Atoche, A.; Ruiz-Ibarra, E.; Osorio De La Rosa, E.; Vázquez Castillo, J. A Self-Powered PMFC-Based Wireless Sensor Node for Smart City Applications. Wirel. Commun. Mob. Comput. 2019, 2019, 1–10.
- Azri, Y.M.; Tou, I.; Sadi, M. Electrodes Materials Evaluation in Plant Microbial Fuel Cells: A Comparison of Graphite and Stainless Steels. Biofuels 2023, 14, 1077–1086.
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the Performance of Constructed Wetland Microbial Fuel Cells Using Three Indigenous South African Wetland Plants. J. Water Process Eng. 2019, 32, 100930.
- Pamintuan, K.R.S.; Sanchez, K.M. Power Generation in a Plant-Microbial Fuel Cell Assembly with Graphite and Stainless Steel Electrodes Growing Vigna Radiata. IOP Conf. Ser. Mater. Sci. Eng. 2019, 703, 012037.
- Huang, X.; Duan, C.; Duan, W.; Sun, F.; Cui, H.; Zhang, S.; Chen, X. Role of Electrode Materials on Performance and Microbial Characteristics in the Constructed Wetland Coupled Microbial Fuel Cell (CW-MFC): A Review. J. Clean. Prod. 2021, 301, 126951.
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.-K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and Recent Trends in Photosynthetic Assisted Microbial Fuel Cells: A Review. Biomass Bioenergy 2021, 148, 106028.
- Zhang, C.; Liang, P.; Jiang, Y.; Huang, X. Enhanced Power Generation of Microbial Fuel Cell Using Manganese Dioxide-Coated Anode in Flow-through Mode. J. Power Sources 2015, 273, 580–583.
- Lv, F.; Zhao, X.; Pan, S.; Cao, W.; Zuo, X.; Li, Y. Electrodeposition of FeOOH Nanosheets on Carbon Felt for Enhanced Sulfamerazine Removal via Visible Light-Assisted Electro-Fenton Process. J. Water Process Eng. 2022, 48, 102883.
- Satar, I.; Daud, W.R.W.; Kim, B.H.; Somalu, M.R.; Ghasemi, M.; Bakar, M.H.A.; Jafary, T.; Timmiati, S.N. Performance of Titanium–Nickel (Ti/Ni) and Graphite Felt-Nickel (GF/Ni) Electrodeposited by Ni as Alternative Cathodes for Microbial Fuel Cells. J. Taiwan Inst. Chem. Eng. 2018, 89, 67–76.
- Türker, O.C.; Baran, T.; Yakar, A.; Türe, C.; Saz, Ç. Novel Chitosan Based Smart Cathode Electrocatalysts for High Power Generation in Plant Based-Sediment Microbial Fuel Cells. Carbohydr. Polym. 2020, 239, 116235.
- Deng, H.; Wu, Y.-C.; Zhang, F.; Huang, Z.-C.; Chen, Z.; Xu, H.-J.; Zhao, F. Factors Affecting the Performance of Single-Chamber Soil Microbial Fuel Cells for Power Generation. Pedosphere 2014, 24, 330–338.
- Lin, F.-Y.; Lin, Y.-Y.; Li, H.-T.; Ni, C.-S.; Liu, C.-I.; Guan, C.-Y.; Chang, C.-C.; Yu, C.-P.; Chen, W.-S.; Liu, T.-Y.; et al. Trapa Natans Husk-Derived Carbon as a Sustainable Electrode Material for Plant Microbial Fuel Cells. Appl. Energy 2022, 325, 119807.
- Narayana Prasad, P.; Kalla, S. Plant-Microbial Fuel Cells—A Bibliometric Analysis. Process Biochem. 2021, 111, 250–260.
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of Modified Chitosan Membrane for Microbial Fuel Cell: Roles of Proton Carrier Site and Positive Charge. J. Clean. Prod. 2017, 142, 1274–1282.
- Sabina-Delgado, A.; Kamaraj, S.K.; Hernández-Montoya, V.; Cervantes, F.J. Novel Carbon-Ceramic Composite Membranes with High Cation Exchange Properties for Use in Microbial Fuel Cell and Electricity Generation. Int. J. Hydrogen Energy 2023, 48, 25512–25526.
- Fan, L.; Shi, J.; Gao, T. Comparative Study on the Effects of Three Membrane Modification Methods on the Performance of Microbial Fuel Cell. Energies 2020, 13, 1383.
- Surti, P.; Kailasa, S.K.; Mungray, A.; Park, T.J.; Mungray, A.K. Vermiculite Nanosheet Augmented Novel Proton Exchange Membrane for Microbial Fuel Cell. Fuel 2024, 357, 130046.
- Sarma, P.J.; Mohanty, K. Development and Comprehensive Characterization of Low-Cost Hybrid Clay Based Ceramic Membrane for Power Enhancement in Plant Based Microbial Fuel Cells (PMFCs). Mater. Chem. Phys. 2023, 296, 127337.
- Saran, C.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Romanholo Ferreira, L.F.; Bilal, M.; Iqbal, H.M.N.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Microbial Fuel Cell: A Green Eco-Friendly Agent for Tannery Wastewater Treatment and Simultaneous Bioelectricity/Power Generation. Chemosphere 2023, 312, 137072.
- Behera, M.; Ghangrekar, M.M. Performance of Microbial Fuel Cell in Response to Change in Sludge Loading Rate at Different Anodic Feed pH. Bioresour. Technol. 2009, 100, 5114–5121.
- Oliveira, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. Overview on the Developments of Microbial Fuel Cells. Biochem. Eng. J. 2013, 73, 53–64.
- Kuleshova, T.E.; Galushko, A.S.; Panova, G.G.; Volkova, E.N.; Appolon, W.; Shuang, C.; Sevda, S. Bioelectrochemical Systems Based on the Electroactivity of Plants and Microorganisms in the Root Environment (Review). Sel’skokhozyaistvennaya Biol. 2022, 57, 425–440.
- Borker, M.; Suchithra, T.V. Rice Paddy as a Source of Sustainable Energy in India. In Proceedings of the 7th International Conference on Advances in Energy Research; Bose, M., Modi, A., Eds.; Springer: Singapore, 2021; pp. 383–392, ISBN 9789811559549.
- Letcher, T.M. Introduction with a Focus on Atmospheric Carbon Dioxide and Climate Change. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–17. ISBN 978-0-08-102886-5.
- Rae, J.W.B.; Zhang, Y.G.; Liu, X.; Foster, G.L.; Stoll, H.M.; Whiteford, R.D.M. Atmospheric CO2 over the Past 66 Million Years from Marine Archives. Annu. Rev. Earth Planet. Sci. 2021, 49, 609–641.
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A.; et al. Recent Global Decline of CO2 Fertilization Effects on Vegetation Photosynthesis. Science 2020, 370, 1295–1300.
- Verma, K.K.; Song, X.-P.; Zeng, Y.; Li, D.-M.; Guo, D.-J.; Rajput, V.D.; Chen, G.-L.; Barakhov, A.; Minkina, T.M.; Li, Y.-R. Characteristics of Leaf Stomata and Their Relationship with Photosynthesis in Saccharum Officinarum Under Drought and Silicon Application. ACS Omega 2020, 5, 24145–24153.
- Gowik, U.; Westhoff, P. The Path from C3 to C4 Photosynthesis. Plant Physiol. 2011, 155, 56–63.
- Rusyn, I.; Hamkalo, K. Electro-Biosystems with Mosses on the Green Roofs. EREM 2020, 76, 20–31.
More