Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mariah Balmaceno-Criss.
Sacral insufficiency fractures are disabling injuries that may occur in the absence of trauma, or result from low-energy injuries such as ground level falls. These fractures can cause severe lower back, buttock, and groin pain and are most commonly identified among older women diagnosed with osteoporosis. Sacroplasty is a minimally invasive procedure for treating sacral insufficiency fractures that involves placement of polymethylmethacrylate cement into the sacral ala.
- sacral insufficiency fractures
- sacroplasty techniques
- short-axis
- long-axis
1. Introduction
Sacral insufficiency fractures are disabling injuries that may occur in the absence of trauma, or result from low-energy injuries such as ground level falls [1]. These fractures can cause severe lower back, buttock, and groin pain and are most commonly identified among older women diagnosed with osteoporosis [2]. Diagnosis can be made using magnetic resonance imaging (MRI), which shows a high-intensity signal indicative of edema on short tau inversion recovery (STIR) sequence. The first line management of these fractures is conservative, consisting of early mobilization, multimodal pain management, activity modification, and osteoporosis management [3]. However, patients that do not respond adequately to nonoperative measures may benefit from sacroplasty or operative fixation [4].
Sacroplasty is a minimally invasive procedure for treating sacral insufficiency fractures that involves placement of polymethylmethacrylate cement into the sacral ala. The cement placement may provide clinically significant pain relief, reducing reliance on pain medication, and improving health-related quality of life [5]. Pain relief can occur in as little as 48 h after surgery, resulting in enhanced functional mobility which, in turn, can reduce the risk of immobility-related complications [6,7][6][7]. Sacroplasty is also a relatively safe procedure with low complication profile. The most common complication is cement leakage [8].
2. Pre-Procedure Preparation
2.1. Patient Selection
Sacroplasty is generally recommended for patients who have failed conservative management, including bed rest and bracing, have adverse reactions to high-dose analgesics, or are unable tolerate long-term immobilization [9,10,11][9][10][11]. Conservative therapy often results in inadequate pain relief and continued mobility challenges associated with sacral insufficiency fractures. In addition, long-term bedrest can pose significant challenges to frailer patients including deep venous thrombosis, pneumonia, muscular atrophy, fatigue, and others [12,13][12][13]. Sacroplasty, on the contrary, allows for better pain management and earlier return to mobility while avoiding the combined impact of prolonged immobilization. Bone mineral density (BMD) scores should also be considered as part of patient selection. Frey et al., explains that low BMD can lead to chronic nonunion at the fracture site because of impaired ability of osteoporotic bone to heal under strain. Sacroplasty may improve pelvic strength and reduce sacral strain, particularly when pursued in combination with anti-osteoporotic medications [14]. At the same time, it avoids the need for screw and plate fixation in this patient population, which can break through bone and result in loss of fixation [15]. Sacroplasty is contraindicated in patients with uncorrected coagulopathy, local or systemic infection, sacral decubitus ulcers, and allergies to cement [5,16][5][16]. In addition, caution should be exercised in patients with gaping fracture lines extending into a sacral foramen or into the dural canal on pre-operative CT since this increases their risk of cement migration into the spinal canal [5].2.2. Classification Systems
Sacral fractures were initially thought to arise from high-energy injuries. As such, they were often classified using the Denis or the AO classification, among others [17]. However, with advanced imaging now showing a higher prevalence of osteoporotic- and stress-related sacral fractures, management based solely on these classification systems is no longer widely agreed upon. This is because these systems emphasize high-energy traumatic mechanisms, often with associated neurologic or pelvic ring injuries, that may warrant more aggressive interventions such as sacroiliac screws or spinopelvic fixation, than sacral insufficiency fractures [18]. Appropriate decision-making between surgical or non-surgical management requires an understanding that sacral insufficiency fractures frequently present in older patients with low bone mineral density following low energy injuries and, as such, create progressive instability from the accumulation of additional fractures [19]. Classification schemes unique to this population have thus been generated. Rommens and Hofmann proposed the fragility fractures of the pelvis (FFP) classification to guide management of fractures occurring anywhere in the pelvis [19]. The FFP classification has four main types with fracture displacement being a key distinguishing factor between the types (Figure 1). FFP Type I often deals with fractures of the anterior pelvic ring while FFP Types II-IV encompass posterior fractures with and without concomitant anterior fractures. FFP Type II encompasses nondisplaced fractures while Types III and IV encompass unilateral and bilateral displaced posterior fractures, respectively. This system recommends conservative treatment for FFP Type I and II fractures [20,21][20][21]. However, close monitoring is recommended for Type II as supplemental percutaneous fixation with screw placement or sacroplasty may be required. FFP Types III and IV necessitate more aggressive operative management and may be augmented with sacroplasty.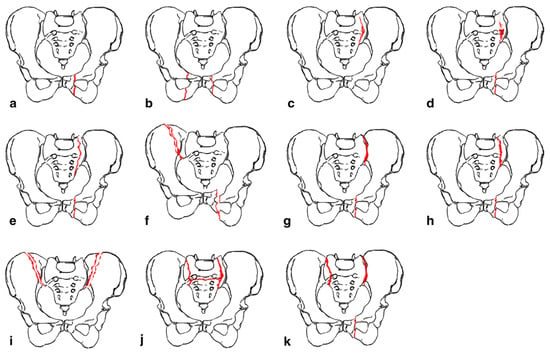
Figure 1. “ Classification of fragility fractures of the pelvis. (a) FFP Type Ia: unilateral anterior pelvic ring disruption. (b) FFP Type Ib: bilateral anterior pelvic ring disruption. (c) FFP Type IIa: dorsal non-displaced posterior injury only. (d) FFP Type IIb: sacral crush with anterior disruption. (e) FFP Type IIc: non-displaced sacral, sacroiliac or iliac fracture with anterior disruption. (f) FFP Type IIIa: displaced unilateral ilium fracture and anterior disruption. (g) FFP Type IIIb: displaced unilateral sacroiliac disruption and anterior disruption. (h) FFP Type IIIc: displaced unilateral sacral fracture together with anterior disruption. (i) FFP Type IVa: bilateral iliac fractures or bilateral sacroiliac disruptions together with anterior disruption. (j) FFP Type IVb: spinopelvic dissociation with anterior disruption. (k) FFP Type IVc: combination of different posterior instabilities together with anterior disruption” by Rommens et al. (Accessed 12 December 2023 at https://doi.org/10.1007/s00776-014-0653-9). Licensed under CC BY-NC-ND 4.0 © 2014 The Japanese Orthopaedic Association. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/ (Red color indicates fracture lines).
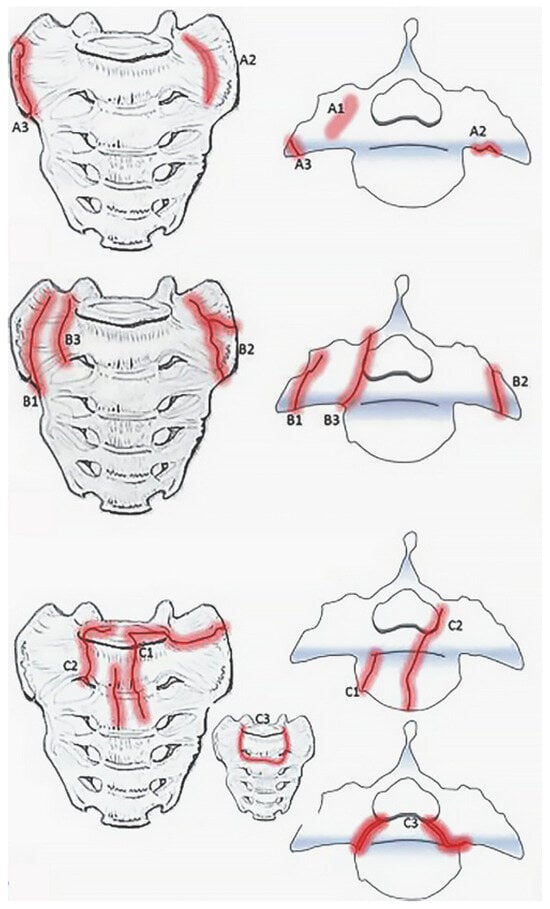
Figure 2. Bakker classification for sacral insufficiency fractures adapted from “Type A-fractures of the sacral ala: A1 with bone bruise (MRI) without a visible fracture line in the CT-scan; A2 deformation of the anterior cortical bone without a cortical disruption; and A3 anterolateral rim fracture of the ala with up to 1 cm distance in the direction of the medial sacroiliac joint.” (top), “ Type B fractures of the sacral ala: B1 fracture parallel to the sacroiliac joint; B2 fracture involving the sacroiliac joint; and B3 fracture with an involvement of the neural foramina or the spinal canal.” (middle), and “ Type C- or corpus-fractures: C1 fracture moves from anterior cortex dorsally or into the sacroiliac joint; C2 fracture with an unilateral involvement of the neural foramina or the spinal canal; and C3 is unstable and represents bilaterally sagittal fractures combined with a transverse lesion.” (bottom) by Bakker et al. (https://doi.org/10.3340/jkns.2017.0188). Original figures licensed under CC BY-NC-ND 4.0© 2018 The Korean Neurosurgical Society. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/ (Red indicates fracture lines).
2.3. Pre-Procedural Optimization
Pain management is an essential part of both conservative and operative management of sacral insufficiency fractures. Multimodal pain management should be pursued to avoid over utilization of potent opioids in this patient population [24]. Additionally, medical optimization of bone health with anti-osteoporotic medications prior to operative intervention should also be considered. For instance, bisphosphonates can be administered to osteoporotic patients awaiting sacroplasty. Bisphosphonates increase early bone growth and improve bone mineral density, which can reduce the risk of vertebral fractures post-operatively [25]. Furthermore, many patients who suffer sacral insufficiency fractures are also vitamin D deficient and may benefit from pre-operative vitamin D supplementation, which has been shown to help reduce the risk of pseudoarthrosis [26]. Finally, hormonal supplementation can improve post-operative healing, reduce bone pain, and analgesic reliance. Calcitonin has been shown to reduce osteoporotic bone pain and is typically used acutely for this purpose [27]. While calcitonin is anti-osteoporotic, its effects are minimal, so it is not typically used as first-line treatment for long-term medical management [28]. Instead, teriparatide, an osteoanabolic agent that is a synthetic form of human parathyroid hormone, has been utilized in postmenopausal women with osteoporosis [29]. Teriparatide promotes new bone formation and remodeling through activation of osteoblasts [30]. Use of teriparatide has been shown to reduce pain, facilitate early mobilization, and promote direct healing in patients undergoing sacroplasty, without increasing rates of primary bone malignancies which has previously been a concern with this medication [31,32][31][32]. Denosumab and Romosozumab, other osteoanabolic agents, have shown an even greater BMD improvement at the lumbar spine and hip through blockade of the inhibitory effects of sclerostin, which results in an increase in bone formation and decrease in bone resorption [33,34][33][34]. Multiple studies have demonstrated superiority of Romosozumab compared to Denosumab for improving BMD at twelve months [35]. However, compared to Denosumab, treatment with Romosozumab is limited to 12 months and cessation is associated with rapid loss of its effects on BMD [36,37][36][37]. Thus, it is recommended that patients begin another antiresorptive therapy after Romosozumab discontinuation. Despite this limitation, treatment with Romosozumab prior to other antiresorptive medications has been shown to result in greater gains in BMD, making this treatment sequence favorable [38]. The use of one or a combination of these medication classes prior to and in conjunction with sacroplasty may help improve patient outcomes.2.4. Patient Positioning
In preparation for sacroplasty, patients are placed in the prone position, with a pillow under the pelvis to elevate the sacrum. Bony landmarks, including the L5-S1 disc, S1 and S2 neuroforamina, and sacroiliac joint, are marked using conventional fluoroscopy. Visualization of these landmarks on fluoroscopy is demonstrated in patient case one. Computed tomography and/or navigation can then be utilized to better visualize the sacral anatomy.2.5. Material Considerations
Materials required for sacroplasty, at bare minimum, include spinal needles, polymethylmethacrylate (PMMA) bone cement, and cement application tools. Use of balloon-assistance, discussed below, may also be considered. The biomechanics of cement injection and the associated risk of cement leakage depend on cement viscosity. A small and safe amount of high-viscosity cement, achieved by increasing the time elapsed since mixing or the powder-to-liquid ratio, injected using a small diameter needle yields the lowest risk of cement leakage [8]. Cumulative procedural costs, not accounting for operating room time and other hospital costs, are approximately $5521–$5784 [39,40][39][40].References
- Urits, I.; Orhurhu, V.; Callan, J.; Maganty, N.V.; Pousti, S.; Simopoulos, T.; Yazdi, C.; Kaye, R.J.; Eng, L.K.; Kaye, A.D.; et al. Sacral Insufficiency Fractures: A Review of Risk Factors, Clinical Presentation, and Management. Curr. Pain Headache Rep. 2020, 24, 10.
- Blake, S.P.; Connors, A.M. Sacral insufficiency fracture. Br. J. Radiol. 2004, 77, 891–896.
- Babayev, M.; Lachmann, E.; Nagler, W. The controversy surrounding sacral insufficiency fractures: To ambulate or not to ambulate? Am. J. Phys. Med. Rehabil. 2000, 79, 404–409.
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P.V. Sacral insufficiency fractures: Current concepts of management. Osteoporos. Int. 2006, 17, 1716–1725.
- Pommersheim, W.; Huang-Hellinger, F.; Baker, M.; Morris, P. Sacroplasty: A Treatment for Sacral Insufficiency Fractures. AJNR Am. J. Neuroradiol. 2003, 24, 1003–1007.
- Butler, C.L.; Given, C.A.; Michel, S.J.; Tibbs, P.A. Percutaneous Sacroplasty for the Treatment of Sacral Insufficiency Fractures. Am. J. Roentgenol. 2005, 184, 1956–1959.
- Gupta, A.C.; Chandra, R.V.; Yoo, A.J.; Leslie-Mazwi, T.M.; Bell, D.L.; Mehta, B.P.; Vanderboom, T.L.; Rabinov, J.D.; Larvie, M.; Hirsch, J.A. Safety and effectiveness of sacroplasty: A large single-center experience. AJNR Am. J. Neuroradiol. 2014, 35, 2202–2206.
- Bastian, J.D.; Keel, M.J.B.; Heini, P.F.; Seidel, U.; Benneker, L.M. Complications related to cement leakage in sacroplasty. Acta Orthop. Belg. 2012, 78, 100–105.
- Chandra, V.; Wajswol, E.; Contractor, S.; Shukla, P.; Kumar, A. 04:12 PM Abstract No. 304 Safety and efficacy of sacroplasty for sacral fractures: A systematic review and meta-analysis. J. Vasc. Interv. Radiol. 2019, 30, 1845–1854.
- Longhino, V.; Bonora, C.; Sansone, V. The management of sacral stress fractures: Current concepts. Clin. Cases Miner Bone Metab. 2011, 8, 19–23.
- Jay, B.; Ahn, S.H. Vertebroplasty. Semin. Interv. Radiol. 2013, 30, 297–306.
- Mahmood, B.; Pasternack, J.; Razi, A.; Saleh, A. Safety and efficacy of percutaneous sacroplasty for treatment of sa-cral insufficiency fractures: A systematic review. J. Spine Surg. 2019, 5, 365–371.
- Kehler, D.S.; Theou, O.; Rockwood, K. Bed rest and accelerated aging in relation to the musculoskeletal and cardio-vascular systems and frailty biomarkers: A review. Exp. Gerontol. 2019, 124, 110643.
- Frey, M.E.; DePalma, M.J.; Cifu, D.X.; Bhagia, S.M.; Daitch, J.S. Efficacy and safety of percutaneous sacroplasty for pain-ful osteoporotic sacral insufficiency fractures: A prospective, multicenter trial. Spine 2007, 32, 1635–1640.
- Acklin, Y.P.; Zderic, I.; Richards, R.G.; Schmitz, P.; Gueorguiev, B.; Grechenig, S. Biomechanical investigation of four different fixation techniques in sacrum Denis type II fracture with low bone mineral density. J. Orthop. Res. 2018, 36, 1624–1629.
- Ortiz, A.O.; Brook, A.L. Sacroplasty. Tech. Vasc. Interv. Radiol. 2009, 12, 51–63.
- Santolini, E.; Kanakaris, N.K.; Giannoudis, P.V. Sacral fractures: Issues, challenges, solutions. EFORT Open Rev. 2020, 5, 299–311.
- Sun, N.; Liu, Y.; Yan, H.; Zhang, Z.; Li, Y.; Zeng, C. Recent Progress in the Classification and Operation of Sacral Fractures. Emerg. Med. Int. 2023, 2023, 2795722.
- Rommens, P.M.; Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 2013, 44, 1733–1744.
- Rommens, P.M.; Ossendorf, C.; Pairon, P.; Dietz, S.-O.; Wagner, D.; Hofmann, A. Clinical pathways for fragility frac-tures of the pelvic ring: Personal experience and review of the literature. J. Orthop. Sci. 2015, 20, 1–11.
- Rommens, P.M.; Arand, C.; Hofmann, A.; Wagner, D. When and How to Operate Fragility Fractures of the Pelvis? IJOO 2019, 53, 128–137.
- Bakker, G.; Hattingen, J.; Stuetzer, H.; Isenberg, J. Sacral Insufficiency Fractures: How to Classify? J. Korean Neurosurg. Soc. 2018, 61, 258–266.
- Bahadir, S.; Nabi, V.; Ayhan, S.; Palaoğlu, S.; Acaroğlu, E. Bakker classification in treatment of sacral stress fractures: A single center experience. J. Turk. Spinal Surg. 2020, 31, 80–84.
- Schwenk, E.S.; Mariano, E.R. Designing the ideal perioperative pain management plan starts with multimodal an-algesia. Korean J. Anesthesiol. 2018, 71, 345–352.
- Byun, J.-H.; Jang, S.; Lee, S.; Park, S.; Yoon, H.K.; Yoon, B.-H.; Ha, Y.-C. The Efficacy of Bisphosphonates for Prevention of Os-teoporotic Fracture: An Update Meta-analysis. J. Bone Metab. 2017, 24, 37–49.
- Mayo, B.C.; Massel, D.H.; Yacob, A.; Narain, A.S.; Hijji, F.Y.; Jenkins, N.W.; Parrish, J.M.; Modi, K.D.; Long, W.W.; Hrynewycz, N.M.; et al. A Review of Vitamin D in Spinal Surgery: Deficiency Screening, Treatment, and Outcomes. Int. J. Spine Surg. 2020, 14, 447–454.
- Chau, A. The Role of Calcitonin for Fracture Pain: Analgesia Effect in as Little as Two Weeks. Can. Pharm. J. 2006, 139, 46–50.
- Pavone, V.; Testa, G.; Giardina, S.M.C.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological Therapy of Osteoporosis: A Systematic Current Review of Literature. Front. Pharmacol. 2017, 8, 803.
- Yang, Y.-C.; Hsieh, M.-H.; Chien, J.-T.; Liu, K.-C.; Yang, C.-C. Teriparatide treatment shows faster healing than sacroplasty for postmenopausal women with sacral insufficiency fracture. Osteoporos. Sarcopenia 2023, 9, 27.
- Haas, A.V.; LeBoff, M.S. Osteoanabolic Agents for Osteoporosis. J. Endocr. Soc. 2018, 2, 922–932.
- Yoo, J.-I.; Ha, Y.-C.; Ryu, H.-J.; Chang, G.-W.; Lee, Y.-K.; Yoo, M.-J.; Koo, K.-H. Teriparatide treatment in elderly patients with sacral insufficiency fracture. J. Clin. Endocrinol. Metab. 2017, 102, 560–565.
- McDonald, C.L.; Johnson, K.; Alsoof, D.; Molino, J.; Balmaceno-Criss, M.; Daniels, A.H. Treatment of Osteoporosis with Anabolic Agents and the Risk of Primary Bone Cancers: A Study of 44,728 Patients Treated with Teriparatide and Abaloparatide. J. Am. Acad. Orthop. Surg. 2023, 31, 520–528.
- Park, H.-Y.; Ha, K.-Y.; Kim, K.-W.; Rhyu, K.-W.; Kim, Y.-H.; Lee, J.-S.; Kim, S.-I.; Park, S.-B. Unstable Bony Chance Fracture Successfully Treated with Teriparatide in Patient with Ankylosed Spine: A Case Report and Review of the Literature. Geriatr. Orthop. Surg. Rehabilitat. 2021, 12, 21514593211039024.
- Lim, S.Y.; Bolster, M.B. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des. Dev. Ther. 2017, 11, 1221–1231.
- Hu, M.; Zhang, Y.; Guo, J.; Guo, C.; Yang, X.; Ma, X.; Xu, H.; Xiang, S. Meta-analysis of the effects of denosumab and romosozumab on bone mineral density and turnover markers in patients with osteoporosis. Front. Endocrinol. 2023, 14, 1188969.
- McClung, M.R.; Brown, J.P.; Diez-Perez, A.; Resch, H.; Caminis, J.; Meisner, P.; A Bolognese, M.; Goemaere, S.; Bone, H.G.; Zanchetta, J.R.; et al. Effects of 24 Months of Treatment with Romosozumab Followed by 12 Months of Denosumab or Placebo in Postmenopausal Women with Low Bone Mineral Density: A Randomized, Double-Blind, Phase 2, Parallel Group Study. J. Bone Miner. Res. 2018, 33, 1397–1406.
- Prather, C.; Adams, E.; Zentgraf, W. Romosozumab: A first-in-class sclerostin inhibitor for osteoporosis. Am. J. Health Syst. Pharm. 2020, 77, 1949–1956.
- Cosman, F.; Kendler, D.L.; Langdahl, B.L.; Leder, B.Z.; Lewiecki, E.M.; Miyauchi, A.; Rojeski, M. Romosozumab and antiresorp-tive treatment: The importance of treatment sequence. Osteoporos Int. 2022, 33, 1243–1256.
- Andresen, R.; Radmer, S.; Wollny, M.; Andresen, J.R.; Nissen, U.; Schober, H.-C. CT-guided cement sacroplasty (CSP) as pain therapy in non-dislocated insufficiency fractures. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 1045–1050.
- Balling, H.; Holzapfel, B.M.; Böcker, W.; Arnholdt, J. Resource Consumption and Remuneration Aspects in Navigated Screw Fixation Procedures with or without Additional Sacroplasty for Fragility Fractures of the Sacrum-A Prospective Clinical Study. J. Clin. Med. 2022, 11, 6136.
More
