You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Paraskevi Kalofiri and Version 2 by Jason Zhu.
Endocrine disruptors (EDs) are molecules capable of mimicking the natural hormones of the body and interfering with the endocrine system in both humans and wildlife. Cosmetic products are one source of EDs; these include an extensive variety of personal care and beauty products designed for the skin and hair, as well as makeup. The widespread use of such products has raised concerns about the presence of EDs within them.
- cosmetics regulation
- endocrine disruptors
- risk assessment
1. Risk Regulation
Science plays a dual role as both the primary creator of environmental problems and as the essential tool for identifying and solving them. This dual role reflects the absolute dependence of societies on science on the one hand, and skepticism towards it on the other, leading to scientific uncertainty [1][16].
The identification of a chemical substance as an ED by the Commission is indeed based on scientific data. However, due to existing scientific uncertainty, a significant role is given to the precautionary principle, which is a fundamental principle in EU risk governance [2][17]. In the EU, the precautionary principle is a cornerstone legal and policy principle with significant application in the field of chemical substances [3][18].
Specifically, it is at the core of the institutional architecture of EU risk regulation, and it is applied at both the risk assessment stage and the risk management stage. During the risk management stage, in line with this principle, the decision-making political body is obligated to establish measures aimed at achieving a high level of environmental and human health protection. This principle allows for action to be taken even in cases where scientific evidence is uncertain or incomplete in order to prevent potential harm to the environment and/or human health [4][19]. It emphasizes the importance of acting cautiously when there are indications of potential harm, even in the absence of conclusive scientific evidence [5][20]. In particular, it allows for the adoption of precautionary measures when scientific data concerning risk to the environment or human health is uncertain and not definitive [6][21]. This means that in cases where the assessment of the risk level (risk assessment) for certain substances with regard to human health or the environment is uncertain, precautionary actions can and should be taken to mitigate potential risks [7][22].
Risk regulation is a process that involves a set of parameters which include the legislative framework, regulatory provisions of the administration, scientific knowledge, and specific policy objectives. The apparent complexity of risk regulation, the core of which is interdisciplinarity, is the reason for the difficulty in scientific or political analysis [8][23].
2. Risk Assessment
The scientific process of risk analysis consists of four steps, as listed in Table 12 [9][10][26,27].
| Hazard identification: | Determining the adverse effects, if there is a potential cause for concern regarding health when individuals are exposed to biological, chemical, or physical agents [11][28]; collecting and evaluating toxicity data from testing systems, epidemiological studies, incident reports, and field observations [12][29]. |
| Dose–response assessment: | Defining how the level of exposure to a substance relates to the likelihood or seriousness of harmful effects occurring in a population exposed to that substance. Thisentails analyzing how the risk of adverse effects varies with varying levels of exposure to a specific substance or agent. This assessment is essential in establishing safe exposure thresholds and offers vital insights in order to make decisions related to risk management and regulations [13][30]. |
| Exposure assessment: | Identifying chemical substances that raise concerns for the exposed population, determining the route through which exposure occurs, and evaluating the magnitude, duration, and timing of doses individuals may have received during their exposure. In other words, it assesses the intensity, frequency, and duration of human exposure to a specific agent [14][31]. |
| Risk characterization: | Synthesizing information gathered in the previous three stages of risk assessment to assess the potential health impacts on the exposed population under various conditions. The goal is to make the risk understandable to relevant authorities and stakeholders, facilitating their understanding of the risk and its implications [15][32]. |
3. The Stages of Risk Assessment
Despite the fact that the general approach to quantitative risk assessment (QRA) has remained largely unchanged since the early 1980s, it is continually evolving in various forms, and its fields of application have significantly expanded. Several organizations and research groups have developed or adopted systematic reviews in the assessment of chemical substances. Moreover, there is a growing body of research which focuses on dynamic risk assessment and risk management, rather than static or traditional risk assessment [16][33]. The use of systematic reviews can identify differences in how questions are formulated, searches are conducted, or studies are evaluated [17][34]. The application of these methods can lead to improved transparency, objectivity, and communication in risk assessment [18][35].On the other hand, the process also involves disadvantages, mainly uncertainty resulting from contentious comparative results, such as the assumption that exposure to high doses applies equally to low doses, and that short-term exposures apply equally to long-term ones. Additionally, it often disregards the synergy of multiple sources of exposure (e.g., chemical substances and their mixtures in real-life exposures). Factors such as these which lead to uncertainty in the risk assessment process are illustrated in Figure 1 [19][20][36,37]. While scientific knowledge is essential, it is not always adequate for the assessment of risks. In any case, the management of risks falls under the jurisdiction of the competent political bodies of the community, which determine whether a risk is acceptable or not [21][38].
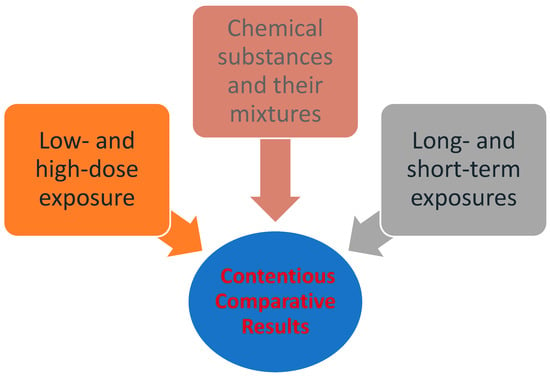
Figure 1. Factors that lead to uncertainty in the risk assessment process [22] (modified by Dr. P.Kalofiri).
4. The Criteria for Determining ED
The criteria for identifying and determining EDs can be found in the regulations for Biocides 2100/2017 [23][40] and Plant Protection Products 2018/605 [24][41], and in the REACH Regulation [25][42]. Substances that pose endocrine disruption risks should not be placed on the market.
The criteria for determining the properties of EDs in humans are different from those that apply to non-target organisms. Both sets of criteria are further subdivided into two sections: one section for defining an ED, and one section regarding the information that must be taken into account for the determination of the properties of EDs [26][43].
In March 2019, the European Parliament published a study that examined the scientific evidence related to the concept of endocrine disruption, the extent of exposure, the relevant health impacts, and the associated costs [27][44]. It called on the EU to establish regulations governing all types of chemicals that cause endocrine disruptions in order to minimize human exposure. The conclusions included several recommendations to EU political bodies regarding goals, the definition of endocrine disruptors, guidance documents, test development and requirements, the management of endocrine disruptors in specific sectors and various areas, production, use, exposure to endocrine disruptors, and research priorities. The Endocrine Society praised the report. It stated that “the report demonstrates that chemicals causing endocrine disruption pose a serious threat to the health of current and future generations and highlight the need for additional action by policymakers in the EU to address this issue” [28][45].
In October 2020, a significant milestone was reached when the European Commission initiated the process of revising the requirements related to the identification of endocrine-disrupting substances. This important development was achieved by amending the Biocidal Products Regulation (Regulation 2021/525) [29][46]. The modifications take into account the “need to reduce testing on vertebrate animals and the need of a testing strategy and methods for the determination of endocrine disrupting properties of substances” [30][47].
In the context of the regulatory procedure, if the Regulatory Committee does not agree with the draft decision submitted by the Commission, the matter is referred to the Council where, if a majority is not achieved, the decision is ultimately taken by the Commission [31][48]. In this process, there are advantages in avoiding deadlock, but there are also disadvantages. The first is that the right of each member state to determine the level of protection it desires is disregarded; the second is that achieving a majority in the Council is difficult, especially in highly sensitive political issues, so that political decisions are finally made by a body which is not democratically legitimized, such as the Commission. While Regulatory Committees are not considered political committees, in practice they often take on a political character because the delineation between political decisions and techno science assessments is ambiguous [32][49]. A notable example is the case of EDs, in which many legal, political, and ethical controversies arise.
The absence of a universally accepted definition makes the risk assessment of EDs more challenging. Relevant public authorities, stakeholders, and the public should all examine the extent of uncertainty as well as its sources and nature, and consider whether it is due to inherent random occurrences or a lack of knowledge [33][50]. Although consultation and public participation play a crucial role in clarifying elements of uncertainty or ignorance, and lead to more informed decisions, the established criteria are highly restrictive and make it very difficult if not impossible to prove that a substance disrupts the endocrine system, as the high degree of uncertainty does not allow for complete proof. Because of the greater burden of proof of harm, more products will remain on the market, resulting in citizens being exposed to dangerous substances and creating a significant burden on the public health budget [34][51]. In other words, the definition requires such a high level of evidence that it ultimately leads to very few substances being considered EDs [35][52].
