Knee osteoarthritis (KOA) represents a prevalent and formidable challenge in the management and treatment of musculoskeletal disorders and exerts a substantial impact on global health, particularly in the context of the natural aging process. Post-traumatic osteoarthritis (PTOA) emerges as a distinct clinical manifestation within the realm of osteoarthritis and is interwoven within the landscape of sports injuries and trauma of the joints. Within the realm of sports, athletes are frequently exposed to joint injury and acute mechanical loading that sets the biological stage for the development of PTOA.
- osteoarthritis
- post-traumatic osteoarthritis
- mechanical loading
1. Introduction
1.1. Knee Osteoarthritis
1.2. Post-Traumatic Osteoarthritis
2. Mechanotransduction
The knee joint, in its healthy state, functions as a hinge joint, which enables vital movements like flexion and extension through the interaction of articular cartilage, synovial fluid, ligaments, and menisci that allow for smooth and continuous motion of the joint. Articular cartilage, the resilient tissue covering the ends of bones, distributes mechanical forces in order to promote frictionless movement. Ligaments provide stability, while the synovial fluid lubricates and nourishes the joint. Together, these components synergize to coordinate the typical physiological processes within the knee joint that allow for seamless motion and weight-bearing activities. In the intricate milieu of KOA, the role of mechanical loading arises as a pivotal determinant in both the pathogenesis and recovery of the knee joint and is intricately linked to mechanotransduction processes. Mechanotransduction, described as the conversion of mechanical signals into biochemical responses, is paramount to understanding how mechanical loading influences the fate of articular cartilage. In the context of this resviearchw, it is critical to note that KOA pathogenesis is deeply involved with aberrations in mechanical loading, which disrupt the delicate balance of loading force required to maintain joint health. In the widely accepted pathological scenario [37][38][39][40][41][42][40,41,42,43,44,45], supramaximal mechanical forces act as a “trigger” for maladaptive responses in chondrocytes, the cells within articular cartilage [43][46]. Research conducted by Buckwalter and colleagues sheds light on the intricate relationship between mechanical loading and articular cartilage fate [43][46]. Their research emphasizes that both acute impact events and cumulative contact stress initiate the release of reactive oxygen species from mitochondria, leading to chondrocyte death and matrix degradation. Importantly, the study illuminates a substantial difference between PTOA primarily caused by acute intense joint injury and OA resulting from chronic joint instability or incongruity. Furthermore, the study describes the capacity of joints with advanced PTOA to remodel and improve with appropriate treatment, emphasizing the dynamic nature of the mechanotransduction processes involved in joint health and repair. There is ample evidence in support of Buckwalter’s research [41][44][45][46][47][48][44,47,48,49,50,51], as it is well characterized that high-impact forces exerted on the joint lead to maladaptive responses in chondrocytes and subchondral bone. As a result, chondrocytes may exhibit increased production of matrix-degrading enzymes, leading to cartilage breakdown and the activation of several inflammatory pathway cascades [49][50][51][38,52,53]. In conjunction with their pathogenic effects, mechanotransduction pathways activated by beneficial or appropriate exercise and mechanical forces stimulate the synthesis of essential extracellular matrix components, which fosters an environment that is favorable to tissue repair and injury prevention [41][52][53][54][55][56][57][44,54,55,56,57,58,59]. While promising, the linkage between mechanical loading and KOA progression requires further investigation as a therapeutic option to understand how mechanical loading can induce beneficial regenerative responses in chondrocytes [58][60]. Through this interplay, well-regulated mechanical loading emerges as a key player in the preservation and restoration of joint health in the context of KOA and PTOA.3. Mechanical Factors in PTOA
The mechanical aspects of PTOA pathogenesis involve complex interactions between joint biomechanics, tissue biology, and the body’s capacity for repair and maintenance of joint health. For example, disrupted joint biomechanics, as a result of trauma or sports injury, will disrupt the delicate balance surrounding mechanotransduction pathways. Altering either cartilage synthesis or degradation creates a mismatch that contributes to accelerated joint degeneration [59][60][61,62]. Additionally, progressive changes in load distribution within the joint due to aging or injury, whether it be from misalignment or other contributing factors, can result in abnormal stresses, leading to the breakdown of cartilage and inflammatory responses, as seen in PTOA and KOA alike [52][61][62][54,63,64]. Research has also elucidated several variables that contribute to PTOA progression, such as variations in flexion angles and resistance [29][30][63][29,30,65]. Therefore, it is crucial to understand that PTOA is complex and under active investigation. Mechanical factors, such as the type of motion and loading stress incurred by the joint, significantly influence the severity of PTOA. Previous studies shed light on the complicated relationship between loading stress and PTOA progression. A previous study, utilizing rabbit models subjected to different impact loads, demonstrated that articular cartilage could tolerate single impact loads up to 45% of the joint fracture threshold without significant disruption or degradation [64][66]. These findings emphasize not only the resilience of articular cartilage, but also highlight the potential long-term consequences of acute mechanical injury and provide a reference point for quantifying “excessive” or supramaximal joint loading. Previous studies by D’Lima et al. [65][66][67,68], focusing on knee joint forces and their impact on OA, underscore the critical role of factors like body weight, muscle contractions, and biomechanics in influencing knee forces. The research emphasizes that each additional kilogram in body weight is multiplied two or three times at the knee, contributing to increased joint loading and potentially accelerating arthritis progression. Malalignment of the lower extremity, as previously discussed, is also identified as a factor associated with the progression of osteoarthritis, which is thoroughly characterized [67][68][69,70]. D’Lima et al. [65][67] also examined knee forces during exercise and recreational activities after knee arthroplasty, providing valuable insights applicable to PTOA. Their study demonstrated, like others [69][70][71][71,72,73], that activities such as running, golf, and tennis were found to produce unexpectedly high forces, especially in the leading knee, emphasizing the need for careful consideration of post-traumatic joint health in athletes engaging in such activities. While the correlation between the increased forces incurred during high-load activities and KOA is unclear [72][73][74][74,75,76], it is evident that these activities do predispose an individual to injury of the knee [75][76][77,78], which can contribute to OA progression. Furthermore, investigation into contact stresses in the knee joint during deep flexion activities by Thambyah and colleagues revealed significantly higher peak stresses, especially in the medial compartment, during squatting, a common resistance exercise [77][79]. The study raised concerns about the adequacy of articular cartilage to support high contact stresses during deep flexion and exemplifies the need to consider mechanical factors in common exercises as contributors to the development of PTOA. Wallace and colleagues quantified patellofemoral joint reaction forces and stress during the squat maneuver and found that patellofemoral joint stress increases linearly with increasing knee flexion angle and joint force [78][80]. The addition of external resistance further elevated patellofemoral joint reaction force and stress. The study suggested that limiting terminal joint flexion angles and resistance loads could help minimize patellofemoral joint stress during squatting activities and may hinder osteoarthritis progression. These insights, among those previously discussed, emphasize the importance of considering specific motions and loading conditions in understanding the mechanical factors influencing the severity of PTOA, and exemplify the multifactorial nature of OA progression (Table 1). Prior research, employing cadaver, in vivo, and in vitro animal cartilage, revealed that chondrocyte death can be triggered by impact stress as low as 18 MPa (megapascals). Furthermore, impact stress exceeding 30 MPa was observed to inflict surface damage on cartilage, ultimately contributing to cartilage degradation [34][79][80][81][82][83][84][85][34,81,82,83,84,85,86,87]. Patellofemoral joint stress linearly correlates with joint force [78][80], with the ratio of patellofemoral joint stress to force being about 2.3 MPa per body weight according to previous studies [77][86][79,88]. It suggests that exceeding eight times the body weight in patellofemoral joint forces may lead to potential cellular injury in articular cartilage by surpassing the critical threshold of 18 MPa. Joint forces are commonly quantified in terms of body weight (BW) in exercises (see next section) and can be used as a quantitative measure to evaluate various joint loading conditions to mitigate or prevent OA.|
Study Focus |
Author, Year [Source] |
Study Type |
Number of Patients |
Study Description |
|---|---|---|---|---|
|
Impact of Athletics on Development of PTOA |
Hootman et al., 2007 [26] |
Epidemiological |
182,000 |
Summarizing injury data to identify preventable risk factors for injury prevention strategies |
|
Golightly et al., 2009 [27] |
Epidemiological |
2528 |
Describes prevalence of KOA within retired football players |
|
|
Drawer et al., 2001 [28] |
Epidemiological |
500 |
Determines the prevalence of KOA within retired soccer players |
|
|
Kujala et al., 1995 [31] |
Epidemiological |
117 |
Analyzing the impact of increased mechanical loading during sport on KOA |
|
|
Swärd et al., 2010 [32] |
Epidemiological |
331 |
Compares radiographic structural changes of KOA and PTOA in athletes v non-athletes |
|
|
Epidemiological |
20 |
Investigates the effect of running on cartilage degeneration in athletes |
||
|
Epidemiological |
825 |
Analyzes the risk of KOA development in patients with sports participation and/or injury |
||
|
Epidemiological |
258 |
Evaluates the effect of triathlon training on likelihood of future injury |
||
|
Epidemiological |
535 |
Analyzes the impact of exercise on risk of knee injury and KOA development |
||
|
Epidemiological |
16 |
Analyzes the relationship between training load and injury in football players |
||
|
Epidemiological |
875 |
Investigates the impact of marathon running on prevalence of injuries in athletes |
||
|
Epidemiological |
326 |
Investigates the effect of previous knee injury on risk of future knee injury in soccer players |
||
|
Mechanical Contributors to OA |
Gillquist et al., 1999 [29] |
Literature review |
- |
Summarizes the risk of ligamentous injury for osteoarthritis progression |
|
Epidemiological |
162 |
Analyzes the effect of malalignment in KOA progression |
||
|
Systematic review |
- |
Determines the effect of running on development of KOA |
||
|
Epidemiological |
26 |
Analyzes the long-term changes of the knee via MRI in former long-distance runners |
||
|
Systematic review |
- |
Analyzes the effect of exercise on risk of KOA |
||
|
Epidemiological |
3 |
Investigates the effects of various activities on mechanical loading in the knee joint |
||
|
Literature review |
- |
Synthesizes studies characterizing forces on the knee joint during various exercises |
||
|
In vivo |
- |
Investigates the impact of varied loading conditions on knee cartilage in rabbit models |
||
|
Systematic review |
- |
Analyzes the effect of previous knee injury on PTOA progression |
||
|
Epidemiological |
11,006 |
Characterizes the effect of malalignment on KOA progression |
||
|
Epidemiological |
229 |
Investigates biomechanical predictors of KOA progression |
||
|
Epidemiological |
4435 |
Analyzes the impact of knee pain or previous injury on the likelihood of future injury |
||
|
Systematic review |
- |
Compiles evidence for the role of inflammation in joint injury and PTOA |
||
|
Literature review |
- |
Discusses risk factors and contributing elements to dysfunction in several joints |
||
|
In vivo |
- |
Evaluate effect of lessened mechanical loading on KOA in mouse model |
||
|
Systematic review |
- |
Summarize the biological underpinnings of the effect mechanical loading on KOA |
||
|
In vitro * |
5 |
Investigates the impact of mechanical loading on gene expression within chondrocytes in OA and non-OA patient samples |
||
|
Epidemiological |
237 |
Investigates the impact of alignment on KOA progression |
||
|
Systematic review |
- |
Analyzes the correlation between malalignment and KOA progression |
||
|
Systematic review |
- |
Summarizes current evidence on the effect of hip muscle weakness in KOA patients |
||
|
In vivo |
- |
Analyzes the effect of mechanical loading on subchondral bone, cartilage, and KOA |
||
|
Epidemiological |
38 |
Evaluates the effect of increased mechanical loading of functional scores of KOA patients |
||
|
Milentijevic 2005 [34] |
In vivo |
- |
Investigates the impact of loading stress on rabbit articular cartilage |
|
|
Roos et al., 1998 [30] |
Epidemiological |
123 |
Determining the effect of meniscal surgery/removal on osteoarthritis progression |
|
|
Therapeutic Potential of Mechanical Loading |
Epidemiological |
12 |
Analyzing the impact of strength-training regimen on muscle development |
|
|
In vitro * |
8 |
Investigates gene expression of human chondrocytes following total knee replacement |
||
|
Epidemiological |
45 |
Analyzes the impact of varied training loads on risk of future injury |
||
|
Systematic review |
- |
Investigates the impact of lateral wedge insoles in patients with KOA |
||
|
Epidemiological |
10 |
Analyzes the benefit of knee brace on minimizing force at the knee joint |
||
|
Epidemiological |
10 |
Investigates the impact of knee brace and orthoses in KOA treatment |
||
|
Epidemiological |
8 |
Investigates the effect of malalignment on KOA progression |
||
|
Epidemiological |
102 |
Compares the effects of high and low load training regimens on KOA functional scores |
||
|
Epidemiological |
89 |
Investigates the effect of water training (low load bearing) on KOA progression |
||
|
Epidemiological |
42 |
Analyzes the effect of quadriceps strengthening on KOA functional scores |
||
|
Epidemiological |
41 |
Analyzes variance in water v. land conditions while performing various exercises in KOA |
||
|
Epidemiological |
35 |
Investigates the effect of varied training regimens on risk of future injury |
||
|
Literature review |
- |
Summarizes the impact of loading conditions on risk of injury, and injury prevention |
||
|
Quantification of Mechanical Forces in Rehabilitation Exercises |
In vitro * |
5 |
Quantifies mechanical forces present at knee joint during walking |
|
|
In vitro * |
4 |
Analyzes mechanical forces present on knee joint during various malalignment conditions |
||
|
Computer model |
- |
Utilizes walking simulation to determine the muscles involved in walking |
||
|
In vivo |
- |
Quantifies mechanical forces during compression to elucidate beneficial loading range |
||
|
Systematic review |
- |
Analyzes the mechanical loading force of OKC and CKC exercises at the knee joint |
||
|
Computer models |
- |
Utilizes computer simulated models to analyze forces on the knee joint during leg extension |
||
|
Epidemiological |
10 |
Quantifies mechanical force at the knee joint during squat, knee extension, and leg press |
||
|
Literature review |
- |
Discusses the mechanical forces incurred at the knee joint during the squat |
||
|
Literature review |
- |
Summarizes the mechanical load incurred during the squat |
||
|
Literature review |
- |
Summarizes the mechanical load during various rehabilitation exercises |
||
|
Epidemiological |
15 |
Quantifies patellofemoral joint forces during the squat |
* Denotes that in vitro samples were conducted on clinical patient samples.
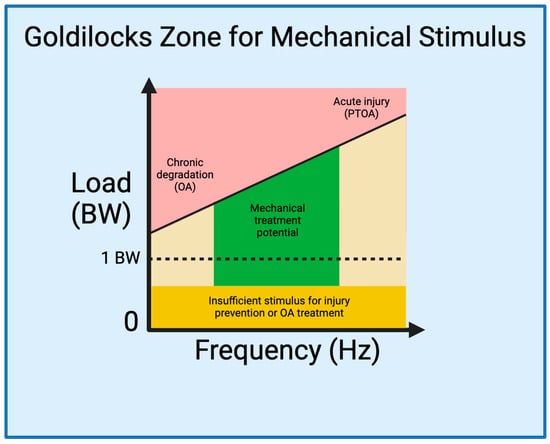
4. The Goldilocks Zone
Establishing a Goldilocks zone of loading that preserves joint health while preventing or mitigating joint injury is an essential step for developing strategies to prevent and treat PTOA (Figure 1). As delineated by Sokoloff’s excellent aphorism in 1969 [118][120], “cartilage can survive in a large range of solicitations, but below or beyond, it will suffer”, illuminating the ideal range of mechanical loading is pivotal for optimizing joint health and the management of PTOA. Studies have described, in-depth and through varied language [119][120][121,122], what thwe researchers are reare referring to as the Goldilocks zone. A 2016 International Olympic Committee (IOC) publication [108][110] critically analyzed the effects of underloading and overloading on athlete injury and performance. Within their discussion, the IOC cites evidence demonstrating the counterbalance between the increased risk of injury in athletes training with sub-competition loads [87][89][90][107][89,91,92,109], and the increased risk of injury with sustained, high-intensity loads [26][88][92][121][122][26,90,94,123,124]. Interestingly, however, the IOC also cites evidence demonstrating a beneficial effect of high-intensity loading on injury prevention [89][98][107][123][91,100,109,125]. This, in essence, demonstrates that high-intensity loading in the Goldilocks zone could prevent joint injury and damage, whereas exhibiting opposite effects outside the zone. Therefore, integrating the current knowledge from mechanobiology studies of knee joints into rehabilitation programs allows us to define the Goldilocks zone as a guide for the development of targeted exercises that optimize beneficial mechanical forces, fostering tissue repair and functional improvement.