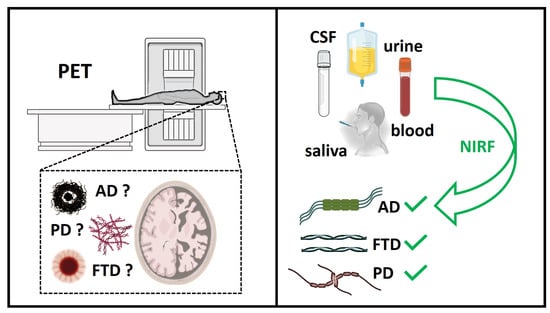
Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) represent two among the most frequent neurodegenerative diseases worldwide. A common hallmark of these pathologies is the misfolding and consequent aggregation of amyloid proteins into soluble oligomers and insoluble β-sheet-rich fibrils, which ultimately lead to neurotoxicity and cell death. After a hundred years of research on the subject, this is the only reliable histopathological feature in our hands. Since AD and PD are diagnosed only once neuronal death and the first symptoms have appeared, the early detection of these diseases is currently impossible. Several reasons could be associated with the lack of effective therapeutic treatments. One of the most important factors is the lack of selective probes capable of detecting, as early as possible, the most toxic amyloid species involved in the onset of these pathologies. In this regard, chemical probes able to detect and distinguish among different amyloid aggregates are urgently needed.
- Aβ1–42
- Tau
- α-synuclein
- probes
- neurodegeneration
- Alzheimer’s disease
- Parkinson’s disease
- tauopathies
1. Introduction
2. Strategies for the Diagnosis of Pre-Symptomatic Neurodegenerative Diseases
References
- Key Figures. Available online: https://institutducerveau-icm.org/en/key-figures/ (accessed on 21 November 2022).
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118.
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. A-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473.
- Karran, E.; De Strooper, B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318.
- Xu, M.; Ryan, P.; Rudrawar, S.; Quinn, R.J.; Zhang, H.; Mellick, G.D. Advances in the Development of Imaging Probes and Aggregation Inhibitors for Alpha-Synuclein. Acta Pharmacol. Sin. 2020, 41, 483–498.
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic Accuracy of 18F Amyloid PET Tracers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385.
- Herholz, K.; Ebmeier, K. Clinical Amyloid Imaging in Alzheimer’s Disease. Lancet Neurol. 2011, 10, 667–670.
- Stenh, C.; Englund, H.; Lord, A.; Johansson, A.-S.; Almeida, C.G.; Gellerfors, P.; Greengard, P.; Gouras, G.K.; Lannfelt, L.; Nilsson, L.N.G. Amyloid-β Oligomers Are Inefficiently Measured by Enzyme-Linked Immunosorbent Assay. Ann. Neurol. 2005, 58, 147–150.
- Hong, M.C.; Kim, Y.K.; Choi, J.Y.; Yang, S.Q.; Rhee, H.; Ryu, Y.H.; Choi, T.H.; Cheon, G.J.; An, G.I.; Kim, H.Y.; et al. Synthesis and Evaluation of Stilbene Derivatives as a Potential Imaging Agent of Amyloid Plaques. Bioorganic Med. Chem. 2010, 18, 7724–7730.
- Sehlin, D.; Söllvander, S.; Paulie, S.; Brundin, R.; Ingelsson, M.; Lannfelt, L.; Pettersson, F.E.; Englund, H. Interference from Heterophilic Antibodies in Amyloid-β Oligomer ELISAs. J. Alzheimer’s Dis. JAD 2010, 21, 1295–1301.
- Mattsson, N.; Lönneborg, A.; Boccardi, M.; Blennow, K.; Hansson, O. Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers Clinical Validity of Cerebrospinal Fluid Aβ42, Tau, and Phospho-Tau as Biomarkers for Alzheimer’s Disease in the Context of a Structured 5-Phase Development Framework. Neurobiol. Aging 2017, 52, 196–213.
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-Synuclein Strains in Parkinson’s Disease and Multiple System Atrophy. Nature 2020, 578, 273–277.
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for Tau Pathology. Mol. Cell. Neurosci. 2019, 97, 18–33.
- Schraen-Maschke, S.; Sergeant, N.; Dhaenens, C.-M.; Bombois, S.; Deramecourt, V.; Caillet-Boudin, M.-L.; Pasquier, F.; Maurage, C.-A.; Sablonnière, B.; Vanmechelen, E.; et al. Tau as a Biomarker of Neurodegenerative Diseases. Biomark. Med. 2008, 2, 363–384.
- Blennow, K.; Hampel, H. CSF Markers for Incipient Alzheimer’s Disease. Lancet Neurol. 2003, 2, 605–613.
- Wallin, Å.K.; Blennow, K.; Zetterberg, H.; Londos, E.; Minthon, L.; Hansson, O. CSF Biomarkers Predict a More Malignant Outcome in Alzheimer Disease. Neurology 2010, 74, 1531–1537.
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, Å.K.; Blennow, K.; Hansson, O. Cerebrospinal Fluid Levels Ofβ-Amyloid 1–42, but Not of Tau, Are Fully Changed Already 5 to 10 Years Before the Onset of Alzheimer Dementia. Arch. General. Psychiatry 2012, 69, 98–106.
- Luk, C.; Compta, Y.; Magdalinou, N.; Martí, M.J.; Hondhamuni, G.; Zetterberg, H.; Blennow, K.; Constantinescu, R.; Pijnenburg, Y.; Mollenhauer, B.; et al. Development and Assessment of Sensitive Immuno-PCR Assays for the Quantification of Cerebrospinal Fluid Three- and Four-Repeat Tau Isoforms in Tauopathies. J. Neurochem. 2012, 123, 396–405.
- Barthélemy, N.R.; Fenaille, F.; Hirtz, C.; Sergeant, N.; Schraen-Maschke, S.; Vialaret, J.; Buée, L.; Gabelle, A.; Junot, C.; Lehmann, S.; et al. Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res. 2016, 15, 667–676.
- Ashton, N.J.; Hye, A.; Rajkumar, A.P.; Leuzy, A.; Snowden, S.; Suárez-Calvet, M.; Karikari, T.K.; Schöll, M.; La Joie, R.; Rabinovici, G.D.; et al. An Update on Blood-Based Biomarkers for Non-Alzheimer Neurodegenerative Disorders. Nat. Rev. Neurol. 2020, 16, 265–284.
- Karikari, T.K.; Ashton, N.J.; Brinkmalm, G.; Brum, W.S.; Benedet, A.L.; Montoliu-Gaya, L.; Lantero-Rodriguez, J.; Pascoal, T.A.; Suárez-Calvet, M.; Rosa-Neto, P.; et al. Blood Phospho-Tau in Alzheimer Disease: Analysis, Interpretation, and Clinical Utility. Nat. Rev. Neurol. 2022, 18, 400–418.
- Kac, P.R.; Gonzalez-Ortiz, F.; Simrén, J.; Dewit, N.; Vanmechelen, E.; Zetterberg, H.; Blennow, K.; Ashton, N.J.; Karikari, T.K. Diagnostic Value of Serum versus Plasma Phospho-Tau for Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 65.
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood Phosphorylated Tau 181 as a Biomarker for Alzheimer’s Disease: A Diagnostic Performance and Prediction Modelling Study Using Data from Four Prospective Cohorts. Lancet Neurol. 2020, 19, 422–433.
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-Tau181 in Alzheimer’s Disease: Relationship to Other Biomarkers, Differential Diagnosis, Neuropathology and Longitudinal Progression to Alzheimer’s Dementia. Nat. Med. 2020, 26, 379–386.
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma Phospho-Tau181 Increases with Alzheimer’s Disease Clinical Severity and Is Associated with Tau-PET and Amyloid-PET. Alzheimer’s Dement. 2018, 14, 989–997.
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma P-Tau231: A New Biomarker for Incipient Alzheimer’s Disease Pathology. Acta Neuropathol. 2021, 141, 709–724.
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic Value of Plasma Phosphorylated Tau181 in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Nat. Med. 2020, 26, 387–397.
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-Tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781.
- Bayoumy, S.; Verberk, I.M.W.; den Dulk, B.; Hussainali, Z.; Zwan, M.; van der Flier, W.M.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Vanbrabant, J.; et al. Clinical and Analytical Comparison of Six Simoa Assays for Plasma P-Tau Isoforms P-Tau181, P-Tau217, and P-Tau231. Alzheimer’s Res. Ther. 2021, 13, 198.
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau Biomarkers in Alzheimer’s Disease: Towards Implementation in Clinical Practice and Trials. Lancet Neurol. 2022, 21, 726–734.
- Balhara, N.; Devi, M.; Balda, A.; Phour, M.; Giri, A. Urine; a New Promising Biological Fluid to Act as a Non-Invasive Biomarker for Different Human Diseases. URINE 2023, 5, 40–52.
- Harpole, M.; Davis, J.; Espina, V. Current State of the Art for Enhancing Urine Biomarker Discovery. Expert. Rev. Proteom. 2016, 13, 609–626.
- Kohlhase, K.; Frank, F.; Wilmes, C.; Koerbel, K.; Schaller-Paule, M.A.; Miles, M.; Betz, C.; Steinmetz, H.; Foerch, C. Brain-Specific Biomarkers in Urine as a Non-Invasive Approach to Monitor Neuronal and Glial Damage. Eur. J. Neurol. 2023, 30, 729–740.
- Shi, M.; Sui, Y.-T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary Tau Species Are Potential Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 299–305.
- Marksteiner, J.; Defrancesco, M.; Humpel, C. Saliva Tau and Phospho-Tau-181 Measured by Lumipulse in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 1014305.
- Pekeles, H.; Qureshi, H.Y.; Paudel, H.K.; Schipper, H.M.; Gornistky, M.; Chertkow, H. Development and Validation of a Salivary Tau Biomarker in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 11, 53–60.
- Ashton, N.J.; Schöll, M.; Heurling, K.; Gkanatsiou, E.; Portelius, E.; Höglund, K.; Brinkmalm, G.; Hye, A.; Blennow, K.; Zetterberg, H. Update on Biomarkers for Amyloid Pathology in Alzheimer’s Disease. Biomark. Med. 2018, 12, 799–812.
- Sabaei, M.; Rahimian, S.; Haj Mohamad Ebrahim Ketabforoush, A.; Rasoolijazi, H.; Zamani, B.; Hajiakhoundi, F.; Soleimani, M.; Shahidi, G.; Faramarzi, M. Salivary Levels of Disease-Related Biomarkers in the Early Stages of Parkinson’s and Alzheimer’s Disease: A Cross-Sectional Study. IBRO Neurosci. Rep. 2023, 14, 285–292.
- Lau, H.-C.; Lee, I.-K.; Ko, P.-W.; Lee, H.-W.; Huh, J.-S.; Cho, W.-J.; Lim, J.-O. Non-Invasive Screening for Alzheimer’s Disease by Sensing Salivary Sugar Using Drosophila Cells Expressing Gustatory Receptor (Gr5a) Immobilized on an Extended Gate Ion-Sensitive Field-Effect Transistor (EG-ISFET) Biosensor. PLoS ONE 2015, 10, e0117810.
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8, 83–94.
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The Human Saliva Metabolome. Metabolomics 2015, 11, 1864–1883.
- Król-Grzymała, A.; Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int. J. Mol. Sci. 2022, 23, 10123.
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of Tear Fluid Amyloid and Tau Levels with Disease Severity and Neurodegeneration. Sci. Rep. 2021, 11, 22675.
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590.
- Ohm, T.G.; Braak, H. Olfactory Bulb Changes in Alzheimer’s Disease. Acta Neuropathol. 1987, 73, 365–369.
- Attems, J.; Jellinger, K.A. Olfactory Tau Pathology in Alzheimer Disease and Mild Cognitive Impairment. Clin. Neuropathol. 2006, 25, 265–271.
- Passali, G.C.; Politi, L.; Crisanti, A.; Loglisci, M.; Anzivino, R.; Passali, D. Tau Protein Detection in Anosmic Alzheimer’s Disease Patient’s Nasal Secretions. Chemosens. Percept. 2015, 8, 201–206.
- Pahrudin Arrozi, A.; Yanagisawa, D.; Kato, T.; Akatsu, H.; Hashizume, Y.; Kaneda, D.; Tooyama, I. Nasal Extracts from Patients with Alzheimer’s Disease Induce Tau Aggregates in a Cellular Model of Tau Propagation. J. Alzheimer’s Dis. Rep. 2021, 5, 263–274.
