Clostridioides difficile is an anaerobic, spore-forming bacterium associated with intestinal infection, manifesting a broad spectrum of gastrointestinal symptoms, ranging from mild diarrhea to severe colitis. A primary risk factor for the development of C. difficile infection (CDI) is antibiotic exposure. Elderly and immunocompromised individuals are particularly vulnerable to CDI. A pivotal aspect for comprehending the complexities of this infection relies on the utilization of experimental models that mimic human CDI transmission, pathogenesis, and progression. These models offer invaluable insights into host–pathogen interactions and disease dynamics, and serve as essential tools for testing potential therapeutic approaches.
- Clostridioides difficile
- gut
- colitis
- innate immunity
1. Introduction
2. Clostridioides difficile Infection
“Bacillus difficile”, as denominated before, entered the scientific spotlight in 1935 when it was first identified by researchers Hall and O’Toole [9][10]. At that time, this bacterium was considered relatively innocuous, quietly inhabiting the human gut without drawing much attention. However, it was during the 1970s that “Clostridium difficile” began to reveal its pathogenic potential [10][11][12][11,12,13]. Researchers observed a connection between C. difficile and antibiotic-associated diarrhea, as well as pseudomembranous colitis, leading to a surge in research interest [8]. Since then, the bacterium has persisted in its evolutionary trajectory, developing resistance to various antibiotics and producing virulent strains that pose significant challenges to healthcare providers and researchers alike [13][14]. C. difficile is a Gram-positive bacterium characterized by its ability to form spores [14][16]. These spores are the resilient, dormant form of the bacterium, exhibiting the capacity to endure adverse conditions, including high temperatures and sterilizing chemicals [15][17]. Their durability contributes to their persistence in the environment, making CDI prevention and control challenging. Moreover, C. difficile spores play a critical role in CDI recurrence; even after successful CDI treatment, some patients experience a recurrence due to the germination of these spores when favorable conditions arise in the colon, often as a result of disruptions to the normal gut microbiota [14][16][17][16,18,19]. The pathological consequences of CDI are primarily centered in the colon. The disease can manifest across a spectrum of severity, ranging from mild diarrhea to severe pseudomembranous colitis, and in extreme cases, leading to fulminant outcomes [18][19][20][23,24,25]. Specifically, the development of pseudomembranous colitis arises as a sequence of unfolding events leading to colitis: CDI often starts with the ingestion of C. difficile spores, typically encountered in the environment, which then transform into active bacteria after germination within the large intestine (cecum and colon) [14][16]. C. difficile produces two crucial toxins, toxin A (TcdA) and toxin B (TcdB), that specifically target epithelial cells by binding to their respective receptors [5][6][5,6]. Once internalized, these toxins cause the subsequent inactivation of Rho-family GTPases through glucosylation [5][21][22][5,26,27]. This event disrupts the host cell’s cytoskeleton, accelerating cell death [23][24][28,29]. In addition, the emergence of hypervirulent strains, such as the NAP1/B1/027 variant, has emerged as a global public health concern by being associated with more severe and recurrent cases of CDI [25][26][27][28][29][33,34,35,36,37]. Specifically, the PCR ribotype 027 strain, commonly denoted as RT027, distinguishes itself with heightened virulence, characterized by elevated production of TcdA and TcdB toxins [30][38]. Infections with the RT027 strain are known for their high recurrence rates, impacting patient outcomes and placing a significant burden on healthcare systems. Remarkably, RT027 demonstrates resistance to certain antibiotics, notably fluoroquinolones, presenting challenges in treatment and contributing to persistent infections [31][32][39,40]. The current strategy for managing and treating patients with CDI typically involves a multifaceted approach [33][46]. Treatment plans are customized to address the unique clinical condition of each individual, underscoring the crucial role of early diagnosis and intervention for effectively managing the disease. The first step is discontinuing the antibiotic that may have triggered the infection, if applicable, to halt the selective advantage C. difficile gains in an altered gut microbiome. The next crucial step is the administration of specific antibiotics, such as vancomycin or fidaxomicin, to target and eliminate C. difficile bacteria [33][34][35][46,47,48]. Metronidazole is designated as an alternative agent and has been omitted from the treatment of nonsevere CDI since the 2021 guidelines [36][49]. Remarkably, fecal microbiota transplantation (FMT) surprisingly emerges as a highly potent therapeutic approach for treating recurrent or severe CDI [37][38][56,57]. This procedure entails the infusion of fecal matter from a healthy donor into the patient’s gastrointestinal tract, with the overarching goal of reestablishing a balanced gut microbiota. The transplanted microbiome, armed with its diverse arsenal of beneficial microbes, plays a crucial role in efficiently combatting the opportunistic C. difficile [3][39][3,58]. Meta-analyses examining the efficacy of FMT for recurrent, severe, or fulminant CDI consistently indicate promising outcomes [40][41][59,60]. FMT has demonstrated high success rates in preventing recurrent CDI, with significant reductions in recurrence compared to standard antibiotic therapy. Moreover, this therapeutic approach has shown effectiveness in treating severe and fulminant cases, contributing to improved clinical outcomes and reduced mortality rates. However, it is not without its drawbacks and potential risks [42][61]. One of the primary concerns is the lack of long-term safety data, as the consequences of introducing a new, diverse microbial community into a patient’s gut over time remain uncertain. FMT relies on donated fecal matter, which, despite rigorous donor screening and testing, may still carry undetected pathogens or infections that can be transmitted to the recipient [43][44][62,63]. There is also a risk of unintended alterations to the recipient’s microbiome, potentially leading to unexpected health issues [45][46][64,65]. Continuing research and ongoing debates focus on the application and long-term consequences of FMT, emphasizing the need for a thorough evaluation of its risks and benefits.3. Animal Models of CDI
CDI has been studied using different animal species, including hamsters, guinea pigs, rabbits, mice, and rats [47][9]. The hamster model is the most commonly used experimental model. On hamsters, CDI can be induced after ingestion of antibiotics, and colonization occurs through experimental challenge or environmental exposure to C. difficile [48][49][50][66,67,68]. The disease in this model primarily affects the cecum with some involvement of the ileum, resulting in diarrhea and fatal enterocolitis when exposed to toxigenic strains [51][52][69,70]. It is important to note that this model represents well the severe and lethal forms of the disease, but does not consistently display the full spectrum of CDI symptoms seen in humans [48][49][53][66,67,71]. Mice and rats are less susceptible to CDI than hamsters [54][73]. Nevertheless, murine models serve as valuable tools for studying CDI, given their genetic similarity to humans and enhanced translational relevance [55][74]. Mouse application allows researchers to control various factors such as genetics, environment, and diet, ensuring consistent experimental conditions. The availability of transgenic mice facilitates the examination of host factors and components of immune responses throughout the progression of the disease. There are well-established protocols for inducing C. difficile infection in mice, making them a standardized and widely adopted model in the field. Typically, laboratory mice are subjected to antibiotic treatment to disturb their gut microbiota, rendering them susceptible to the germination and colonization of C. difficile—mirroring the dynamics observed in humans [56][57][75,76]. Then, the mice are orally inoculated with C. difficile spores or vegetative cells. Over time, infected mice may develop symptoms similar to those seen in humans, such as diarrhea, weight loss, and other gastrointestinal issues [58][59][54,77]. The severity of symptoms varies depending on the C. difficile strain used and the susceptibility state of the mouse [60][78]. Numerous mouse models have been created for studying the mechanisms behind C. difficile pathogenesis, and each has its own strengths for investigating different aspects of the disease [56][75]. The selection of a particular method is guided by the research goals, the specific strain of C. difficile, and the intended disease features. One common approach is to perform a single oral gavage with C. difficile spores, as it mimics the natural route of infection from contaminated surfaces [61][62][80,81]. To study recurrent infections, some models involve multiple rounds of challenge [58][63][50,54]. Alternatively, researchers can administer purified C. difficile toxins to study the role of toxins in disease pathogenesis [64][65][66][67][82,83,84,85]. Antibiotic pretreatment disrupts the gut microbiota, making mice more susceptible to colonization, reflecting conditions seen in patients with antibiotic-associated C. difficile disease [68][69][70][86,87,88]. Gnotobiotic/germ-free mice can be colonized by C. difficile and exhibit intestinal pathology, primarily in the colon, with pseudomembrane formation similar to human disease [71][72][73][74][92,93,94,95]. This model in particular enables the exploration of microbiota or isolated groups of bacteria’s role in the development of CDI. Reeves et al. unveiled a significant delay in the onset of primary CDI and relapse in germ-free mice pretreated with probiotics [75][96]. Notably, the microbiome of mice undergoing moderate CDI and receiving probiotic treatment revealed a striking increase in the abundance of the Lachnospiraceae family during the initial CDI phase. Intriguingly, mice that were precolonized with the Lachnospiraceae isolate demonstrated a substantial decrease in C. difficile colonization, decreased levels of intestinal cytotoxin, and exhibited milder clinical symptoms and colonic histopathology. This effect was not observed in mice solely colonized with E. coli as a control. Theis study suggests novel therapeutic approaches for the treatment and prevention of CDI by utilizing bacterial species that potentially inhibit C. difficile growth. Likewise, a bacterial consortium named VE303 is currently under development as a potential treatment for high-risk CDI [76][77][97,98]. This oral treatment consists of eight nonpathogenic, nontoxigenic, commensal strains of Clostridia that have been shown to effectively restore a healthy gut microbial community, mitigate inflammation, and elevate levels of protective metabolites in mice [76][77][97,98]. However, the use of gnotobiotic mouse models has a much higher cost and is less practical when compared to conventional mice. Additionally, the absence of other microorganisms in these models makes them less representative of the human situation. Nonetheless, the utilization of germ-free mice is crucial for investigating the specific role of human microbiota in the development of CDI. Humanized mouse models, engrafted with human microbiota, provide an environment closer to human C. difficile infection [78][79][80][81][99,100,101,102].4. Antibiotic-Induced Murine Model of CDI
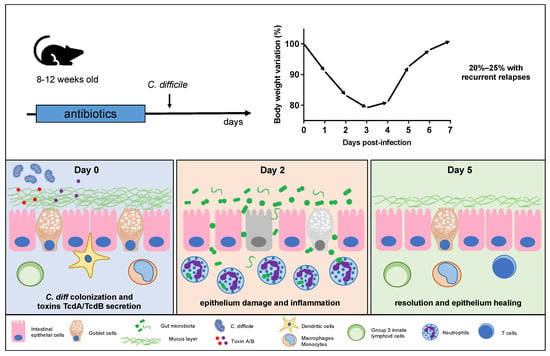
Antibiotic-induced murine models of CDI are specifically designed to mimic the clinical manifestations and pathological features of human CDI within a controlled laboratory setting [61][82][80,103]. Despite some limitations, this model has greatly contributed to our understanding of CDI and continues to be an essential component of CDI research. Antibiotics are employed to initiate gut dysbiosis, a crucial step in simulating the clinical conditions that lead to C. difficile expansion [69][83][84][87,104,105]. Commonly used antibiotics for this purpose include vancomycin, cefoperazone, and tetracycline, typically administered orally via drinking water supplementation [54][85][86][87][88][73,106,107,108,109]. The selection of antibiotics varies according to the specific study goals and the desired level of gut dysbiosis, with the administration of these antibiotics for a predetermined period before introducing C. difficile to initiate infection. Giel et al. conducted a study on the antibiotic-induced mouse model of CDI to investigate C. difficile spore germination [16][18]. Their findings revealed that spores were more likely to germinate in antibiotic-treated mice, but this germination was mitigated by cholestyramine, a treatment that chelated bile salts. This observation led the authors to suggest that cecal bacterial populations in antibiotic-treated mice had a reduced capacity to modify taurocholate, a spore germinant factor, providing further support for the role of bile salts in C. difficile spore germination and infection development in the host [16][18]. In some research scenarios, a combination of antibiotics may be utilized to induce a more profound disturbance in the gut microbiota, closely mirroring the clinical situation where patients often receive multiple antibiotics before developing CDI. Chen et al. [61][80] pioneered an innovative mouse model wherein C57Bl/6 mice were subjected to a 3-day oral exposure to a combination of kanamycin, gentamicin, colistin, metronidazole, and vancomycin, followed by an intraperitoneal administration of clindamycin 48 h later. Subsequently, the mice were challenged with varying doses of C. difficile. This mouse model faithfully replicated human C. difficile infections, inducing characteristic symptoms such as diarrhea, weight loss, and typical histological features. The severity of the disease in this mouse model was proportional to the challenge dose used, ranging from 2 × 102 to 105 CFU.5. CDI Pathogenesis and Disease Progression in Mice
After antibiotic treatment, the mouse’s gut undergoes substantial modifications, rendering it susceptible to C. difficile colonization [69][89][90][87,116,117]. The highly resilient C. difficile spores colonize the intestines, primarily finding their niche in the murine cecum and colon [14][91][16,118]. Within these regions, spores undergo germination, transitioning into vegetative cells that actively grow and produce the toxins responsible for clinical symptoms (Figure 1) [91][118]. Toxin A (TcdA) and toxin B (TcdB) are released following C. difficile germination, typically occurring on the first day postinfection [91][118]. These toxins have a specific affinity for the intestinal epithelial cells, disrupting the colonic epithelium and subsequently contributing to tissue damage and bacterial translocation [92][93][94][95][119,120,121,122]. In particular, gut bacterial translocation involves the process through which bacteria, mostly those that naturally inhabit the intestinal tract, cross the protective barrier of the gut and enter the bloodstream or other distant anatomical regions.