You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by CONSTANTINOS GIAGINIS.
Diet and dietary supplements aim to add trace elements, vitamins, and minerals to the body to improve human health and boost the immune system. In the peculiar circumstances of the COVID-19 pandemic, in combination with disease prevention techniques, the strengthening of the immune system is considered particularly important to enable it to effectively respond to and eliminate the SARS-CoV-2 viral pathogen in the event of infection.
- COVID-19 pandemic
- dietary supplements
- dietary patterns
- vitamins
- trace elements
- omega-3 fatty acids
- Mediterranean diet
- probiotics
- infection
- virus
1. Introduction
1.1. Dietary Supplements
Food supplements can act as adjuvants and may contribute to supplementing the normal diet without being a substitute for food. They are concentrated sources of nutrients marketed in various dosage forms (capsules, lozenges, tablets, pills, powder sachets, sachets of liquid products, vials of liquid products, dropper bottles, etc.). Dietary supplements do not require FDA approval for their safety and efficacy because they are not therapeutic drugs but only essential components for promoting the protection of immunological, metabolic, physical, and mental development [12][1]. Diverse supplements and nutrients, which were investigated for their beneficial effects on viral infections [13,14,15,16][2][3][4][5] have sparked the interest of the scientific community in an effort to adopt preventive and effective measures and treatments against COVID-19 [4][6].
There are also several types of herbal supplements/natural products which have been considered as they may act against diverse infections, including COVID-19 [17][7]. The most widely used are ginger, garlic, honey, turmeric (curcumin), lemon, black seed, cinnamon, and anise. However, there are not sufficient research data thus far suggesting that they could provide any benefit against COVID-19 infection and disease symptoms’ severity [17][7]. A previous systematic review identified 12 randomized controlled trials examining diverse herbal medicine interventions on individuals suffering from COVID-19 [18][8]. Although some of the included studies provided preliminary evidence that herbal supplements may contribute to the recovery of COVID-19 patients, most of them suffered from severe methodological biases [18][8]. Thus, they should be taken into careful consideration because of their overall low quality, highlighting the strong demand to perform more well-organized clinical trials.
1.2. SARS-CoV-2
The World Health Organization (WHO) received a report of an unexplained pneumonia outbreak at the end of 2019 in Wuhan, China. Following virus isolation from bronchoalveolar lavage fluid samples from individuals with pneumonia symptoms, the viral RNA sequence was determined, and this infectious agent was identified as beta-coronavirus, a previously unknown coronavirus type [19][9]. In January 2020, the WHO recognized an increased incidence of serious acute respiratory syndrome triggered by coronavirus 2 as an epidemic condition. The International Committee for the Classification of Viruses identified SARS-CoV-2 as the causal factor; however, in February 2020, the WHO reclassified this disorder as COVID-19 (ICTV) [20][10].
SARS-CoV-2 constitutes the third consecutive zoonotic human respiratory coronavirus of the twenty-first century to damage the respiratory system and represent a serious hazard for public health, next to the introduction of SARS-CoV in 2002 and MERS-CoV in 2012, respectively. To maintain normal function and improve the immune system, it is essential to consume sufficient vitamins and minerals, especially when dealing with disorders such as COVID-19 infection [21][11].
2. SARS-CoV-2 and Dietary Supplements
Diverse clinical studies have been conducted to examine the potential beneficial effects of diet patterns and dietary supplements against the COVID-19 pandemic. The most important beneficial effects and the underlining mechanisms of diet patterns and dietary supplements are depicted in Figure 21.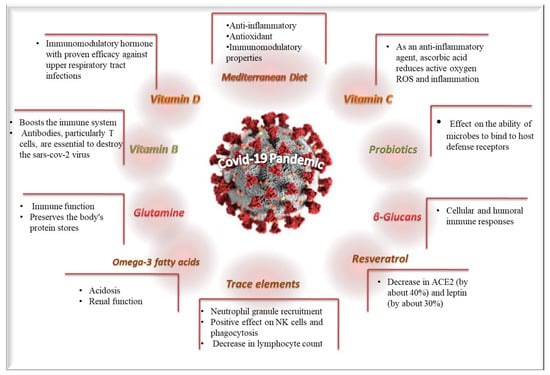
Figure 21.
The most important beneficial effects and the underlining mechanisms of diet and dietary supplements against COVID-19.
2.1. Trace Elements
Zinc (Zn) constitutes a trace element having a recommended daily intake of 15 mg, which participates in the innate and developed response to viral infections [22][12]. Zn is involved in neutrophil granule recruitment and can exert a beneficial impact on NK cells and phagocytosis [23][13]. Notably, Zn deficiency is associated with a decrease in lymphocyte count. Zn supplementation also raises the amount of T and NK cells, as well as enhancing interleukin (IL)-2 expression. With respect to SARS-CoV-2, it was found to suppress the biosynthesis, reproduction, and transcription system of coronaviruses. Zn supplementation additionally appears to suppress symptoms associated with lower respiratory tract infection (as in the case of COVID-19) [23][13]. In this context, a recent clinical study conducted on 88 COVID-19-positive children patients and 88 healthy children showed that the Zn mean concentrations of the COVID-19 positive children were substantially decreased compared to the healthy children [24][14]. In addition, a case-control clinical study including 93 adult patients suffering from COVID-19 and 186 healthy adults with no COVID-19 symptoms also revealed that serum Zn concentrations were decreased in COVID-19-positive adults than the healthy individuals [25][15]. However, it should be emphasized that Zn supplementation above the recommended doses may exert harmful side effects like copper deficiency and medication absorption decrease [26][16]. Thus, it remains uncertain whether Zn supplementation could be safely and effectively used against COVID-19 infection. In agreement with the previous studies, a systematic review by Balboni et al. identified only a small number of surveys examining the potential impacts of Zn supplements against COVID-19 pathology, which did not verify efficacy [27][17]. Notably, this systematic review found 22 unpublished forthcoming clinical trials, 19 on Zn, 1 on selenium (Se), and 2 for both of them. Thus, Balboni et al. concluded that although the preventive or co-therapeutic Zn or Se supplementation interventions against COVID-19 are at present unjustified, the findings of the ongoing studies will be published, and this could alter the currently existing conclusions [27][17]. Selenium (Se) with a recommended daily intake of 35 μg constitutes an essential trace element, which exerts a crucial impact as a redox agent since it occurs in the form of selenocysteine in the catalytic centers of many selenoproteins. The synthesis of selenocysteine requires a continuous resource of the amino acid serine. Se deficiency potentially affects both forms of the immunological response concerning viruses’ pathogenesis [28][18]. Remarkably, oxidative stress together with Se deficiency works synergistically in converting the virus from mild to highly infectious. In this aspect, Se as an antioxidant agent plays a crucial role as a cofactor in a family of enzymes which functions adjunctively with vitamin E to decrease reactive oxygen species known as ROS. An impaired antioxidant defense may herald an excessive inflammation response to the host similar to non-active infection [28][18]. The most potent antioxidant Se-dependent enzymes include glutathione peroxidases (GPXs) and thioredoxin reductases (TXNRDs) that require Se to be functional. Other selenoproteins such as selenoprotein K (SELENOK) and selenoprotein S (SELENOS) are related to immunoregulation. Another factor that cooperates with these seleno-dependent enzymes is the cofactor Q10. This supplement appeared to reduce the specific inflammation response, especially concerning infections such as COVID-19 that exhibit hyperactive inflammation; however, there is no clinical study thus far for such an action of cofactor Q10 [28][18]. A population-based, retrospective study found a relation of the cure COVID-19 rates with Se status [29][19]. However, this study had several limitations preventing sufficient conclusions from being drawn [29][19]. In this aspect, a critical review study reported that Se levels may affect the human response to SARS-CoV-2 pathology. Moreover, Se status could be one among the various risk factors that could affect the outcome of SARS-CoV-2 disease, mainly in individuals with low or suboptimal Se consumption [30][20]. However, at this time, there is no substantial study to support whether Se status may be related with COVID-19 pathology. Magnesium (Mg) with a recommended daily intake of 350 mg constitutes an important essential trace element and the main cation of human cells localized in mitochondria. Mg has already been used in lung diseases characterized by inflammation such as asthma and pneumonia. Specific studies have demonstrated that Mg sulphate enhanced the bronchodilation induced by an administered β2 agonist in individuals with a chronic obstructive lung disorder [31][21]. Patients with COVID-19 experienced respiratory tract symptomatology, most notably cough, sore throat, nasal congestion, and dyspnea. Mg sulfate can act as a calcium antagonist that usually finds application in suppressing bronchial smooth muscle fiber reduction and stimulating bronchodilation [31][21]. In this aspect, hypomagnesemia and hypermagnesemia might have major negative consequences, perhaps by progressing from a moderate to a severe development of COVID-19. Subjects presenting reduced Mg concentrations could be more prone to develop and enhance this illness. There is insufficient evidence thus far concerning the Mg status of COVID-19 patients presenting varying intensity of symptoms’ severity [30][20]. In this context, a cross-sectional retrospective survey on 1064 individuals with COVID-19 staying in a hospital showed that a Mg-to-Ca ratio ≤ 0.20 was highly associated with death rates in individuals with progressed COVID-19 disease [32][22]. Iron (Fe) with a recommended daily intake of 15 mg is a common mineral, which plays a crucial role for the enzymes’ activity of immune cells, like neutrophils and lymphocytes, while serious Fe shortage has been related to a high risk of diverse infections [33][23]. On the contrary, excessive Fe levels have been found to raise the probability of several infections [34][24]. In this aspect, it should be noted that COVID-19 has been shown to affect Fe homeostasis, while a pilot, retrospective cohort survey in 50 individuals with COVID-19 staying in a hospital showed a relation between decreased serum Fe concentrations and COVID-19 related death rates and symptoms’ intensity [35,36][25][26]. This is a first clinical study, which highlights the strong demand to perform future well-designed clinical studies on this topic.2.2. Vitamin A
Vitamin A has a recommended daily intake of 600 μg and it is essential for the consistent immunity, exerting a crucial impact in the growing of T cells, T helper cells, and B cells. In this aspect, a study by Rohani et al. investigated the role of vitamin A intake on the therapeutic effect of patients presenting COVID-19 [32][22]. Both groups under study received the standard anti-COVID-19 drug therapy, and one of the groups orally received 25,000 IU/d of vitamin A for 10 days. Clinical symptoms in the two groups did not differ significantly before the intervention. However, there were significant differences in reduction in fever, body pain, exhaustion, and white blood cell count for the experimental group [32][22]. Even if there is not any other study thus far, this first study seems promising and should trigger the performance of clinical studies in the future, which could reveal positive impacts of vitamin A against COVID-19 risk infection and/or symptoms’ disease severity.2.3. Vitamins B Complex
The normal function of the immunological system and energy metabolism, as well as cell function, all depend on the vitamin B complex [56][27]. A lack of the vitamin B complex may cause the cytokine levels which reinforce immune system function, particularly innate and adaptive immunological responses, to decline [56][27]. Its connection to SARS-CoV-2 viral infection and the possibility of utilizing it as a dietary supplement should therefore be evaluated [57][28]. Cardiovascular issues, neuroinflammation, increases in inflammation, and aberrant antibody responses might be considered as consequences of thiamine deficiency. Thiamine (vitamin B1, recommended daily intake: 1.4 g) shortage could result in insufficient antibody responses, which may subsequently exacerbate disease symptoms [56,58][27][29]. This is ascribed to the fact that antibodies, particularly T cells, are essential to destroy the SARS-CoV-2 virus. In addition, thiamine acts as a carbonic anhydrase isoenzyme suppressor. Consequently, it is anticipated that adequate thiamine levels will maintain healthy immunological responses during SARS-CoV-2 infection, and that substantial dosages of thiamine administered to patients during the initial steps of COVID-19 infection could minimize hypoxia and stays in the hospital [56][27]. When riboflavin (vitamin B2, recommended daily intake: 1.6 mg) and ultraviolet (UV) light are mixed, irreparable damage is caused to nucleic acids like DNA and RNA, inhibiting the reproduction of microorganisms. Both riboflavin and UV light have demonstrated adequate efficacy against MERS-CoV, proposing that they may additionally be beneficial against SARS-CoV-2. In a study conducted by Ragan et al., plasma and blood units that had previously been contaminated with SARS-CoV-2 were subjected to treatment with riboflavin and UV radiation [59][30]. The infection load of SARS-CoV-2 dropped below the detection limit with a mean viral load reduction of more than 4.79 ± 0.15 logs in plasma and 3.30 ± 0.26 logs in whole blood units. These findings suggested that the use of riboflavin and UV radiation may partially lower the probability of COVID-19 virus transmission via transfusion and may also decrease the number of viral cells in COVID-19 patients who are critically unwell [59][30]. Even if there is not any clinical study thus far to support the above actions of vitamin B2, this hypothesis seems to have an increased probability of being confirmed in the near future by the performance of well-designed randomized clinical trials. The B3 vitamin, niacin, with a recommended daily intake of 18 mg, serves as a precursor to nicotinamide adenine dinucleotide (NAD)+ and NADP+, both of which are essential during systemic chronic inflammation [60][31]. When NAD+ levels are increased, a variety of pathophysiological disorders can be treated. NAD+ functions as a co-enzyme in diverse metabolism pathways. Niacin (B3) supplementation may also assist COVID-19 patients in reducing the inflammatory process, which is often triggered by IL-6 and is generated during the early stages of inflammatory cascade [60][31]. NAD+ is known to suppress pro-inflammatory cytokines, IL-1, IL-6, and TNF-α. Moreover, the B3 vitamin decreases neutrophil infiltration and has anti-inflammation benefits in individuals presenting ventilator-induced lung damage in intensive health care units. Niacin also appears to protect the lungs and enhance immune function; hence, it may be utilized as a supplementary agent to the existing therapies for COVID-19 patients [60][31]. Folic acid is a water-soluble vitamin with a recommended daily intake of 400 μg that is classified as part of the vitamin B complex (vitamin B9). Folic acid intake is essential for human health due to its preventative effects against megaloblastic anemia and neural tube birth defects, as well as other chronic diseases. Folic acid may be recommended as an adjuvant therapy for COVID-19 and early-stage respiratory disorder due to the fact that tetrahydrofolic acid and 5-methyl tetrahydrofolic acid exhibit considerable and constant binding affinities against SARS-CoV-2 [60][31]. Notably, it has been postulated that elevated dosages of folic acid, 5 mg or higher, could be considered an effective therapeutic approach for pulmonary hypertension and/or refractory severe hypoxemia in individuals presenting serious COVID-19-related pneumonia in which NO treatment is applied [37][32]. In this context, Ibrahimagić et al. have further supported the above hypothesis, reporting that hyperhomocysteinemia could appear in individuals presenting pulmonary hypertension and the concentrations of folic acid are adversely related with those of homocysteine [61][33]. However, there is no clinical trial to test this hypothesis until the results of the above clinical trial to be published. The most bioavailable and active form of vitamin B12, methylcobalamin (recommended daily intake: 6 μg), has been shown by Narayanan and Nair during 2020 [62][34] to exert the potential to lessen organ damage and COVID-19-related symptoms when administered. Positively polarized single-stranded RNA makes up the SARS-CoV-2 viral genome. One of the proteins encoded by the genome is the NSP12 protein, which is essentially in charge of replicating the viral genome. A homology model of the protein has been used to show that methylcobalamin can bind to the NSP12 protein’s active site [62][34]. This vitamin has a great affinity for the region of the viral nucleotide bonds that are being received. Additionally, viral replication is impeded because the vitamin’s binding site coincides with those of the entering nucleotide, as well as the RNA substrate [62][34]. Vitamin B12 exerts a crucial role in promoting red blood cell generation, central nervous system health, myelin formation, cell proliferation, and prompt DNA biosynthesis. Deficiency of vitamin B12 can result in intestinal inflammation, increases in ROS, and oxidative stress. Vitamin B12 can also modulate the intestinal flora. SARS-CoV-2 may interfere with the metabolism of vitamin B12, hence hindering the growth of intestinal flora [60][31]. Therefore, it is conceivable that COVID-19 infection symptoms like enhanced oxidative stress and lactate dehydrogenase, hyperhomocysteinemia, activation of the coagulation cascade, vasoconstriction, and renal and respiratory vasculopathy, are mainly caused by vitamin B12 deficiency. Additionally, a B12 shortage can result in problems with the digestive, respiratory, and brain systems. Because there is a lack of gastric acid or the intrinsic factor required for active B12 absorption, which makes it difficult for them to absorb this vitamin from diet, the elderly may also have the highest vitamin B12 deficit. This condition may contribute to the elderly’s higher sensitivity to COVID-19 infection [60][31]. At this time, an ongoing multicenter, double-blind, randomized, and placebo-controlled phase-IIIb trial investigates the treatment of individuals presenting post-COVID-19-syndrome (PC19S) with prednisolone and/or vitamins B1, B6, and B12 for a duration of 28 days in primary care and it is expected to announce its results in the next few months [63][35]. This clinical trial assesses the viability, protection, and efficiency of treating patients in primary care with prednisolone and/or vitamin B1, B6, and B12 and it will be positive if the advantage of any therapy is proved beyond placebo operationalized via an enhancement of at least three points on the Patient Reported Outcomes Measurement Information System (PROMIS) total score (t-score) [63][35].2.4. Vitamin C
Vitamin C, also well-recognized as ascorbic acid with a recommended daily intake of 75 mg, is a water-soluble vitamin with strong anti-oxidant properties. Vitamin C is unstable and sensitive to heat and air and is destroyed by oxidation. Vitamin C is also oxidized by smoking, so for smokers the proposed daily consumption of vitamin C is significantly greater than for non-smokers. Vitamin C is found in abundance in green leafy vegetables, tomatoes, peppers, citrus fruits, kiwi fruit, and pineapple, as well as in animal organs such as kidneys and liver. Vitamin C has a wealth of immunomodulatory, anti-inflammatory, antioxidant, anti-thrombotic, and antiviral properties. Ascorbic acid also appears to be involved in T-lymphocyte maturation, as well as phagocytosis and chemotaxis functions [64][36]. Phagocytes import vitamin C in its oxidized form (dehydroascorbic acid) and transform it into vitamin C (ascorbic acid) and this explains the impact of this vitamin as an anti-oxidant agent. Concerning COVID-19, vitamin C is related to the decrease in several cytokines, so it exerts a key impact in protecting the endothelium from oxidative damage [64][36]. As an anti-inflammatory agent, ascorbic acid reduces active ROS (such as peroxides, oxygen free radicals, hydroxyl radicals, etc.) and inflammation. As a result, ascorbic acid can stop the lungs from being harmed by ROS. A cytokine storm is specifically brought on by COVID-19 infection, which also causes systemic lung inflammation. Nitric oxide (NO) and other inflammatory mediators, including IL-6 and -CSF, are produced by highly activated macrophages as a result of the infection [64][36]. As an immunomodulator, ascorbic acid inhibits the motivation of the nuclear factor (NF)-κβ that plays a central role in overall immunity. An elevated dosage of vitamin C promotes the proliferation of T and B leukocytes and nuclear killer (NK) cells. This strengthening of immune system cells suppresses the cytokine storm and improves host immunity as a whole [64][36]. Furthermore, although SARS-CoV-2 has been shown to reduce the expression of type 1 interferons (IFs), which are indeed the first line of defense of the body against viral infections, ascorbic acid positively regulates the expression of these proteins, thus manifesting its antiviral role [64][36]. In another study, Tehrani et al. examined the co-therapeutic effect of vitamin C in individuals suffering from COVID-19 with mild as well as severe symptoms. The group that received the vitamin C intervention received intravenous ascorbic acid at a dosage of 2 g every 6 h for 5 days in conjunction with the typical therapy [65][37]. The level of oxygen saturation considerably increased in the ascorbic acid group. In addition, the vitamin C group showed a markedly reduced respiratory rate. The computed tomography scans of the lungs of participants in both groups, as stated by radiologists, were also evaluated. After the end of the treatment, the rate of lung involvement in the vitamin C group was much more lower compared to the control group, as reported by the radiologists [65][37]. Furthermore, the study by Tosato et al. combined the use of l-arginine and ascorbic acid administered orally twice daily and demonstrated improved performance in walking, muscle force, endothelium function, and fatigue in adults presenting long COVID-19 [66][38]. One study looking at the impact of elevated intake of ascorbic acid (intravenous) showed that the control group receiving vitamin C stayed in hospital less (about two days less) than the group receiving only the basic treatment [67][39]. In addition, Fogleman et al. suggested that taking nutritional supplements may improve the symptoms and quality of life of individuals presenting minor to medium COVID-19 infection [68][40]. However, comparing vitamin C, melatonin, and placebo, they did not find any difference in the group taking placebo and the group consuming ascorbic acid, while they found improvement in the group receiving melatonin. Specifically, vitamin C administration (1000 mg orally for 14 days) had no effect on disease progression [68][40]. Additionally, a retrospective study examining self-reported frequent nutritional supplement use data from 372,720 UK participants in the initial waves of the pandemic up to 31 July 2020 was performed [38][41]. In general, this study showed that ascorbic acid supplementation did not exert any considerable impact against SARS-CoV-2 infection. A beneficial relationship was only found concerning male participants aged >60 years receiving vitamin C supplements in testing positive for SARS-CoV-2 [38][41]. Accordingly, a randomized clinical trial evaluated whether high-dose ascorbic acid may decrease the symptoms’ intensity or duration in 214 individuals presenting SARS-CoV-2 infection [26][16]. Again, vitamin C supplementation did not significantly lower symptoms’ severity and duration compared with standard care [26][16]. Moreover, a two-center, non-interventional, retrospective cohort study conducted on 296 patients admitted to an intensive care unit (ICU) with a proven COVID-19 infection investigated the potential effect of a low dosage of ascorbic acid as an adjunctive treatment in COVID-19 infection disease [39][42]. Once more, a low dosage of ascorbic acid as an adjunctive treatment against COVID-19 was not related to death rates benefits; however, it was associated with a reduced prevalence of thrombosis [39][42]. Thus, there is insufficient evidence thus far to recommend ascorbic acid supplementation for preventing or treating COVID-19 pathology.2.5. Vitamin D
Vitamin D constitutes a secosteroid hormone with a recommended daily intake of 5 μg, which could minimize COVID-19 negative outcomes by modulating either the renin-angiotensin system (RAS), and the innate and adaptive cellular immunity, or the physical barriers, and the host frailty and comorbidities [69][43]. Vitamin D belongs to the class of fat-soluble hormones and is currently known as a steroid hormone. Two types of vitamin D have been identified: D2 (ergocalciferol) derived mainly from plant sources and D3 (cholecalciferol) derived mainly from animal sources [40][44]. With no effective SARS-CoV-2 treatment, chemoprevention, or immunization, concentrating on the urgent repurposing of current medications offers promise against the COVID-19 pandemic. A novel unbiased genomics-guided tracking of SARS-CoV-2 targets in human cells recognized vitamin D as one of the top three compounds exhibiting putative infection modification patterns. This premise is supported by increasing pre-clinical and epidemiologic observational evidence. In support of this view, Annweiler et al. anticipated that vitamin D administration might enhance COVID-19 prognosis [40][44]. Vitamin D is considered as an immunoregulatory hormone with enhanced effectiveness against upper pulmonary tract infections. Its immunomodulatory activity is directly related to its anti-inflammatory activity. Vitamin D is involved in T-lymphocyte maturation and can divert helper type 17 (Th17) cells to anti-inflammatory, regulatory T cells (T-reg) [40][44]. The proinflammatory cytokines IL-1, IL-6, IL-12, and TNF-α seem to be decreased by this differentiation, whereas the anti-inflammatory IL-10 is increased. Additionally, vitamin D affects the transition of T helper cells from type 1 to type 2 [40][44]. As a result of this migration, pro-inflammatory cytokines are also present in lower quantities. Inhibiting NF-κβ, which is likewise a function of vitamin D, can also reduce these cytokines. As a result, vitamin D can decrease the cytokine cascade associated with COVID-19 through multiple mechanisms. Vitamin D is recommended for use against COVID-19, since it has been demonstrated to lower angiotensin-converting enzyme 2 (ACE2) receptor levels and T cell differentiation. In this aspect, a multicenter, randomized controlled clinical trial in individuals aged ≥65 years and presenting COVID-19 showed that an elevated dosage of vitamin D supplementation could be considered as an efficient, well-tolerated, and simply and directly available co-treatment for COVID-19, the prevalence of which can be increased considerably [41][45]. A multicenter randomized clinical trial was performed to compare the impacts of 5000 IU daily oral vitamin D3 supplementation compared to 1000 IU daily oral vitamin D3 supplementation on symptoms’ and other clinical parameters’ recovery in mild to moderate COVID-19 patients presenting suboptimal vitamin D levels [41][45]. This study found that a lack of vitamin D could be linked to an elevated probability of COVID-19 severity [41][45]. In Tan’s et al. study, it was found that in older individuals with COVID-19, a vitamin D/Mg/vitamin B12 mixture was associated with a considerable reduction in the percentage of patients presenting clinical deterioration and requiring oxygen support, intensive care support, or both of them [42][46]. This research suggests that population-based randomized controlled clinical trials should be conducted to determine the overall impact of this mixture in reducing the symptoms’ intensity of COVID-19 [42][46]. In this aspect, AlSafar et al. found that inadequate blood concentrations of the neurohormone vitamin D are linked to an enhanced probability of symptoms’ intensity and death rates from COVID-19. In spite of the worldwide immunization deployment and good first outcomes, the emphasis remains on additional COVID-19 prevention measures [43][47]. However, vitamin D at a dosage of 800 or 3200 IU/day cannot affect the defensive effectiveness or immunogenicity of SARS-CoV-2 vaccination when administered to adults presenting lower levels of vitamin D at disease onset [44][48]. By collecting and scrutinizing the currently existing clinical data, seven meta-analyses and systematic reviews and three subsequent clinical trials claimed that reduced vitamin D levels enhanced the predisposition to COVID-19 and the probability of higher disorder severity and mortality [70][49]. Moreover, five meta-analyses and systematic reviews have currently examined vitamin D supplementation for its capacity to prevent acute pulmonary infection, as well as COVID-19 [70][49]. A newer randomized controlled clinical trial evaluating vitamin D supplement intervention (52,000 IU monthly) in 198 patients with a 3- to 6-month follow-up of SARS-CoV-2 infections was also performed [45][50]. The above study clearly showed that vitamin D supplementation in patients with 25(OH)D3 concentrations ranging from 20 to 100 ng/mL exhibited a decreased rate of SARS-CoV-2 infection than the usage of nutritional-hygienic measures at 6 months follow-up [45][50]. In addition, a systematic review and meta-analysis of primarily cohort surveys concluded that vitamin D supplementation could be beneficial for the decrease in COVID-19 symptoms’ intensity, including mortality [71][51]. In spite of the above hopeful results, it remains unclear whether vitamin D supplementation may prevent or co-treat of COVID-19 in healthy individuals. However, wone cannot rule out that it could exert beneficial effects in those patients diagnosed with malnourishment, vitamin D insufficiency, or low vitamin D levels.2.6. Omega-3 Fatty Acids
Omega-3 fatty acids with a recommended daily intake of 1000–16,000 mg have demonstrated benefits for the immune system, also exerting strong anti-inflammatory properties [72][52]. Omega-3 fatty acids are well-recognized for their ability to combat viruses such as influenza [73][53]. The oxygenation of COVID-19 patients was shown to be enhanced by omega-3 fatty acids. However, the above evidence should be used with caution, as several items of data reveal an increase in oxidative stress and inflammation in individuals with COVID-19 [74][54]. Numerous scientific and clinical research studies have linked omega-3 fatty acids consumption to improvements in general health, cardiovascular health, eye health, and brain development [46][55]. In addition, it is believed that these fatty acids possess hepatoprotective, neuroprotective, hypolipidemic, and anti-inflammation, as well as antioxidant, activities [75][56]. Several of these research studies explain the immunomodulatory impacts of omega-3 fatty acids and their power to increase and regulate mood, suggesting that omega-3 fatty acids could be capable of boosting mental health, while simultaneously enhancing human immunity against COVID-19 [47,75,76][56][57][58]. Sedighiyan et al. performed a blinded, randomized, controlled clinical trial in a Tehran hospital to determine whether omega-3 fatty acids may be a suitable adjunct to other anti-inflammatory drugs for COVID-19 patients (Amir-Alam) [46][55]. Thirty-three individuals with COVID-19 were allocated into two groups; one received hydroxychloroquine as a placebo and the other received hydroxychloroquine along with 2 g of each omega-3 fatty acid for two weeks [46][55]. Compared to the control group, omega-3-supplemented patients showed a considerable improvement in all clinical symptoms except for bodily pain, tiredness, appetite, and olfactory symptoms. Both c-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) were also reduced by omega-3 supplementation in comparison with the control group following therapy. Nevertheless, after supplementation, no variations in liver enzyme serum concentrations between groups were noted [46][55]. Moreover, a strong disadvantage of the above study was the small number of the enrolled individuals. Several clinical symptoms, including body discomfort, fatigue, and a loss of appetite, improved in the group receiving omega-3 fatty acid supplements, according to the existing findings. The loss of smell did not improve, and there was no difference between groups in the concentration of liver enzymes. According to this study [76][58], a moderate dosage of omega-3 fatty acids may hold promise for reducing COVID-19-induced inflammation-related clinical symptoms [76][58]. Doaei et al. [47][57] also revealed that the interventional group exhibited a significantly greater one-month survival rate, arterial pH, HCO3, and base excess (Be) level, as well as arterial blood urea nitrogen (BUN), creatinine (Cr), and potassium (K) level, than the control group. The blood glucose, sodium (Na), hematocrit test (Hct), Ca, phosphorus (P), mean arterial pressure (MAP), O2sat, PO2, PCO2, white blood cells (WBCs), Glasgow Coma Scale (GCS), hemoglobin (Hb), platelet (Plt), partial thromboplastin time (PTT), and albumin levels were not substantially diverse between the two groups [47][57]. Due to the beneficial effects of omega-3 dietary supplementation on acidosis and renal function, the results of this randomized clinical study suggest that it may improve clinical outcomes in COVID-19-infected individuals. Hence, it is crucial to perform additional clinical investigations, utilizing a variety of omega-3 polyunsaturated fatty acids (PUFAs) dosages, larger sample sizes, and longer time periods [47][57].2.7. Glutamine
Glutamine, with a recommended daily intake of 3–6 g, exerts a crucial impact on several functions of the immunological system. It is a vital source of energy for immunological system cells, such as white blood cells (WBCs) and specific intestinal cells. Additionally, glutamine deficiency can impair the immune system’s ability to function. After severe injuries such as burns, high-protein high-glutamine diets or glutamine supplements are frequently prescribed [48][59]. Glutamine supplements may also improve human health, reducing infections and shortening hospital stays after surgery. In other words, glutamine may significantly contribute to immune function. Nonetheless, during illness or injury, the body may be unable to produce sufficient glutamine amounts. Glutamine supplements can enhance immune function and preserve the body’s protein stores [48][59]. Glutamine supplement treatment for a period of 5 days also considerably enhanced patients’ appetite relative to the control group, and four days of glutamine supplementation significantly reduced serum levels of TNF-a, high-sensitivity CRP, and IL-1 [48][59]. In the above study, patients who agreed to take glutamine comprised the interventional group, which was distinguished from patients in the control group who did not take glutamine [61][33]. The 230 individuals with COVID-19 in the control group were comparable to the 228 individuals with COVID-19 who used l-glutamine in terms of age, gender, and clinical condition [48][59]. The interventional group received 10 gr of glutamine three times daily for a period of 5 days. At the last day of the intervention, additional blood samples were received to measure serum concentrations of IL-1, TNF-a, malondialdehyde, and total antioxidant capacity [48][59]. A decrease in appetite causes a deficiency in protein, zinc, vitamin C, and other micronutrients that boost immunity, which exacerbates the disease’s effects. In comparison to the control group, glutamine supplementation significantly enhanced hunger levels throughout the course of five days in the study’s experimental group. Both IL-1 and TNF-a levels in the blood were considerably reduced after 4 days of glutamine supplementation, according to the findings of this study [48][59]. Moreover, individuals with COVID-19 have higher oxidative stress amounts than healthy people. By ingesting 10 g of glutamine three times daily, COVID-19 patients presenting pulmonary infections can reduce their concentrations of IL-6, TNF-a, and hs-CRP and increase their appetite. Thus, this supplement can aid these patients in avoiding malnutrition by stimulating their appetite [48][59].2.8. Resveratrol
Resveratrol with a recommended daily intake of 50–500 mg belongs to the polyphenols, which have considerable antioxidant properties. It is produced by plants—as with other phytochemicals—in response to pests and UV radiation. In small quantities, it is known to be found in about 70 plant species, mainly in the skin of the fruit. As an antioxidant, it can also reduce the oxidation of ‘bad’ LDL cholesterol, which causes atherosclerosis [49][60]. Resveratrol, in addition to its strong antioxidant property, exerts a beneficial effect on the onset and progression of atherosclerosis, prevents oxidative stress and the formation of reactive oxygen radicals, exerts anti-inflammatory action, inhibits the modification/oxidation of LDL and platelet aggregation, modifies the complications of atherosclerosis, and exerts cancer-preventive properties [49][60]. In addition, according to Marlies de Ligt et al., the cell-surface receptor that allows SARS-CoV-2 to go into the cells is ACE2 [49][60]. Due to the high expression of ACE2 and the resulting increased viral transmission in obese individuals with COVID-19, adipose tissue (AT) is a possible SARS-CoV-2 reservoir. Despite the lack of human investigations, rodent and cell research studies have shown that the polyphenol resveratrol changes ACE2. Marlies de Ligt et al. [49][60] examined whether resveratrol supplementation for a period of 30 days in obese persons could alter renin-angiotensin system (RAS) components in both AT and skeletal muscle. This study was a placebo-controlled cross-over clinical trial. Resveratrol showed a low impact on the expression of angiotensinogen, ACE2, and angiotensin II type 1 receptor (AT1R) in AT or skeletal muscle RAS components, but it considerably decreased ACE2 (by about 40%) and leptin (by about 30%). The above results support evidence that resveratrol treatment decreases ACE2 in AT, which may inhibit the spread of SARS-CoV-2 in COVID-19 [49][60]. Marlies de Ligt et al. also found that resveratrol treatment significantly reduced the gene expression of leptin and ACE2 in central and peripheral AT in comparison with a placebo [49][60]. There were no discernible differences in the expression of the AT genes for AGT, ACE2, and AT1R between the resveratrol group and the control group. Moreover, the expression of the angiotensinogen (AGT) and ACE2 genes in skeletal muscle did not differ significantly from a placebo [77][61]. The above findings showed that resveratrol administration substantially lowers ACE2 and leptin expression in AT. These results may have ramifications concerning obese people, since resveratrol supplementation may reduce their probability of contracting SARS-CoV-2 by decreasing the expression of the ACE2 receptor in AT. Additionally, resveratrol reduced AT leptin expression, which may exert favorable impacts on the outcome of COVID-19. It should be emphasized that after SARSCoV-2 entrance, ACE2 expression is inhibited, which could lead to higher angiotensin II levels and poor clinical outcomes in individuals with COVID-19 [77][61]. Moreover, the COVID-19 pandemic resulted in unhealthy lifestyles, namely a rise in overweight and obesity, which has been associated with an elevated chance of poor COVID-19 outcomes. In this aspect, web-based health services might be proven beneficial, particularly in times of severe constraints [77][61].2.9. Beta-Glucans
Beta glucans are polysaccharides with a recommended daily intake of 200 mg, which are considered as nature’s immunological regulator, reinforcing prime immune cells and enhancing and balancing optimal natural immunity. The beta-1,3-D glucans in cereals help to control glucose and cholesterol levels, enhancing weight loss while protecting the gastrointestinal system. Similarly, the beta-1,6-D glucans in mushrooms shield the immune system. In general, they are considered “functional ingredients” since their regular consumption has been found to exert a beneficial impact on human health [50][62]. Furthermore, 72 volunteers participated in a single-center, randomized clinical trial, where they were either given a placebo or a coactive mixture of yeast-based components with a special 1.3/1.6-glucan complex and a group of heat-treated probiotic Saccharomyces cerevisiae enriched with Se and Zn (ABB C1®) the day after receiving an anti-inflammatory medication, according to Rodriguez et al. [50][62]. For the influenza vaccination group, the treatment plan lasted 30 days, while for the COVID-19 vaccine group, it lasted 35 days. Following the second dosage of the COVID-19 immunization, the mean CD4 + T cell counts in the ABB C1® group were elevated from 910.7 cells/L at baseline to 1000.2 cells/L, but they fell in the placebo group from 1055.1 cells/L to 929.8 cells/L [50][62]. Both CD3 + T and CD8 + T cells underwent the same modifications. In the COVID-19 cohort, the ABB C1® supplement group experienced higher elevations in IgG and IgM than the placebo group. Patients receiving the active medicine experienced higher serum levels of Zn and Se than those receiving a placebo. ABB C1®-related significant adverse events or problems with tolerance were not recorded. Thus, this study’s findings support ABB C1®’s ability to stimulate trained immunity [50][62]. Originally intended to have 45 participants in each cohort, recruitment issues led to the inclusion of 72 participants (27 men and 45 women), 29 of whom (40.2%) were above the age of 70 [50][62]. The influenza vaccine cohort had evaluations at three different times: baseline, 7th day (middle assessment), and 30th day (ultimate assessment). Concerning COVID-19 vaccine cohort, evaluations were carried out at baseline, on Days 7, 21, and 35. ABB C1® (n = 33) or a placebo (n = 34) were administered to 67 out of the total 72 subjects [50][62]. The remaining five subjects violated the protocol by not receiving a vaccination, so they were dropped from the analysis. In total, 32 vaccinated participants received a supplement with ABB C1® (COVID-19, n = 18; influenza, n = 14) and 32 received a placebo (COVID-19, n = 14; influenza, n = 14) among the vaccinated participants who completed the clinical trial [50][62]. Subjects who received the COVID-19 mRNA vaccine from Pfizer-BioNTech, supplementation with ABB C1®, a consortium of S. cerevisiae-glucan complex, and were supplemented with Se and Zn exhibited T-cell responses after a single dosage [50][62]. Compared to the placebo group, the ABB C1®-supplemented group showed higher concentrations of cellular and humoral immune responses, which were not affected by participants’ age or gender. According to this recent study, antibody markers showed a clear association with vaccine effectiveness, as well as a high negative association with COVID-19 risk [50][62].2.10. Probiotics
Probiotics with a recommended daily intake of 109–1010 colony forming units (CFUs) are tiny microorganisms that deliver several health benefits to their host. The major place of action for probiotics is the large intestine. Hence, a probiotic must avoid the negative impacts of stomach acid and the relevant digestive enzymes to access the large intestine. Once in place, probiotics provide their purported benefits by promoting the growing and metabolic activities of the bacteria that ferment them [78][63]. The main general target bacteria for probiotic activity are the biphospho-bacteria and lactobacilli, although this may change as knowledge of microbial diversity and functionality expands [79][64]. It cannot, however, be excluded that probiotics have a direct effect on human health, e.g., through immune system mechanisms [79][64]. Several studies have examined COVID-19 patients’ gut microbiotas; however, nothing is known about the dynamics of the resistome, a gene bank for antimicrobial resistance (ARGs). The effects of antibiotics or probiotics on the ARGs reservoir were shown in the study by Qi Su et al. (2022), which examined the potency of the resistome from the time of diagnosis to 6 months following viral clearance [51][65]. They profiled the longitudinal fecal metagenomes of 142 individuals with COVID-19 infection. At baseline, antibiotic-naive COVID-19 patients had ARGs that were more abundant, diversified, and ubiquitous than non-COVID-19 controls. Tetracycline, vancomycin, and multidrug-resistant genes were the primary drivers of resistome-expansion for at least 6 months next to SARS-CoV-2 elimination [51][65]. Those presenting larger resistomes were more susceptible to the post-acute COVID-19 syndrome and Klebsiella sp. Throughout the acute infection and recovery stage of COVID-19, oral probiotics (synbiotic formula, SIM01) considerably lowered the ARGs reservoir in the intestinal microbiome. On the other hand, antibiosis treatment increased the amount of ARGs. Overall, these findings provide new light on the potency of the ARGs reservoir in COVID-19 patients and promote the expectancy that microbiome-facilitated treatment may support patients with COVID-19 that have accumulated ARGs, decreasing their burden [51][65]. Furthermore, between 1 March 2020 and 31 January 2021, 24 patients who had already received treatment and 66 individuals presenting proven SARS-CoV-2 infection were assigned [51][65]. They were monitored for as long as 9 months after the virus was contained. Before and after contracting SARS-CoV-2, each of these individuals went at least 6 months without antibiotic therapy. Also, 66 non-COVID-19 individuals with the same age, sex, and comorbidities as the individuals with COVID-19 were enrolled [51][65]. Using metagenomic profiling, the resistome of a feces sample was analyzed. Using Bray–Curtis dissimilarities, these researchers were able to distinguish between the resistomes of baseline samples from individuals with COVID-19 and without COVID-19. In samples of individuals with COVID-19, the diversity and number of discovered ARGs were significantly higher. By normalizing to the overall number of 16S reads, the total abundance of ARGs in samples from individuals with COVID-19 remained significantly higher compared with the samples from individuals without COVID-19 [51][65]. Knowing what affects the human intestinal resistome, as well as how to inhibit resistome proliferation, means fighting AMR is easier [51][65]. This study uses information from shotgun metagenomic sequencing of samples from various human populations to show whether probiotics and antibiotics could affect the resistome. When COVID-19 was admitted, they observed a sizeable development of the resistome, which continued to grow throughout the virus-positive interval and remained for months after virus clearance [51][65]. Although using antibiotics, the virus was present and additional aided in the development of the resistome, taking an over-the-counter probiotic (Synbiotic Formula, SIM01) prior to or after virus clearance, which was related with a decrease in the amount and abundance of ARGs accumulated in individuals with COVID-19. Moreover, no rebound effect was noted throughout the 12-week follow-up [51][65]. A randomized, multicenter clinical trial has recently been performed in 200 patients with a complaint of post-COVID-19 fatigue [52][66]. The interventional group (n = 100) consumed the oral probiotic supplements for 14 days and the non-interventional group (n = 100) consumed a placebo. The probiotics’ supplemental intervention led to the resolution of fatigue in a higher proportion of patients of the interventional group than the non-interventional group on 14th day. Patients in the interventional group also exhibited a substantially higher decrease in the overall, as well as the physical and mental fatigue, scores at all time points compared to the non-interventional group [52][66]. Collectively, the currently available evidence concerning the impacts of probiotics’ supplementation on preventing COVID-19 infection and disease symptoms’ severity seem promising but not quite adequate. Further population-based clinical studies should be performed to derive conclusive results.References
- Maughan, R.J.; King, D.S.; Lea, T. Dietary supplements. J. Sports Sci. 2004, 22, 95–113.
- Zhang, L.; Liu, Y. Potential Interventions for Novel Coronavirus in China: A Systematic Review. J. Med. Virol. 2020, 92, 479–490.
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094.
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65.
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717.
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, e00122-21.
- Arora, I.; White, S.; Mathews, R. Global Dietary and Herbal Supplement Use during COVID-19-A Scoping Review. Nutrients 2023, 15, 771.
- Feng, Z.; Yang, J.; Xu, M.; Lin, R.; Yang, H.; Lai, L.; Wang, Y.; Wahner-Roedler, D.L.; Zhou, X.; Shin, K.M.; et al. Dietary supplements and herbal medicine for COVID-19: A systematic review of randomized control trials. Clin. Nutr. ESPEN 2021, 44, 50–60.
- Yadav, P.D.; Sarkale, P.; Razdan, A.; Gupta, N.; Nyayanit, D.A.; Sahay, R.R.; Potdar, V.; Patil, D.Y.; Baradkar, S.; Kumar, A.; et al. Isolation and Characterization of SARS-CoV-2 Beta Variant from UAE Travelers. J. Infect. Public Health 2022, 15, 182–186.
- Raghavan, K.; Dedeepiya, V.D.; Suryaprakash, V.; Rao, K.-S.; Ikewaki, N.; Sonoda, T.; Levy, G.G.; Iwasaki, M.; Senthilkumar, R.; Preethy, S.; et al. Beneficial effects of novel aureobasidium pullulans strains produced beta-1,3-1,6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study. Biomed. Pharmacother. 2022, 145, 112243.
- Razzaque, M.S. COVID-19 Pandemic: Can Zinc Supplementation Provide an Additional Shield against the Infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378.
- Science, M.; Johnstone, J.; Roth, D.E.; Guyatt, G.; Loeb, M. Zinc for the Treatment of the Common Cold: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. CMAJ 2012, 184, E551–E561.
- Balboni, E.; Zagnoli, F.; Filippini, T.; Fairweather-Tait, S.J.; Vinceti, M. Zinc and Selenium Supplementation in COVID-19 Prevention and Treatment: A Systematic Review of the Experimental Studies. J. Trace Elem. Med. Biol. 2022, 71, 126956.
- Doğan, A.; Dumanoğlu Doğan, İ.; Uyanık, M.; Köle, M.T.; Pişmişoğlu, K. The Clinical Significance of Vitamin D and Zinc Levels with Respect to Immune Response in COVID-19 Positive Children. J. Trop. Pediatr. 2022, 68, fmac072.
- Elham, A.S.; Azam, K.; Azam, J.; Mostafa, L.; Nasrin, B.; Marzieh, N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 276–282.
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs. Usual Care on Symptom Length and Reduction among Ambulatory Patients with SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369.
- SeyedAlinaghi, S.; Shahidi, R.; Mojdeganlou, H.; Akhtaran, F.K.; Maroufi, S.F.; Maroufi, S.P.; Mirzapour, P.; Karimi, A.; Khodaei, S.; Pour, M.M.; et al. The effect of macronutrient and micronutrient supplements on COVID-19: An umbrella review. J. Health Popul. Nutr. 2024, 43, 16.
- Golin, A.; Tinkov, A.A.; Aschner, M.; Farina, M.; da Rocha, J.B. Relationship between Selenium Status, Selenoproteins and COVID-19 and Other Inflammatory Diseases: A Critical Review. J. Trace Elem. Med. Biol. 2023, 75, 127099.
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299.
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627.
- Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramírez-Renteria, C.; Martínez-Aguilar, G.; Marrero-Rodríguez, D.; Ferreira-Hermosillo, A.; Simental-Mendía, L.E.; Remba-Shapiro, I.; Gamboa-Gómez, C.I.; et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients 2022, 14, 1686.
- Rohani, M.; Mozaffar, H.; Mesri, M.; Shokri, M.; Delaney, D.; Karimy, M. Evaluation and Comparison of Vitamin a Supplementation with Standard Therapies in the Treatment of Patients with COVID-19. East. Mediterr. Health J. 2022, 28, 673–681.
- Kumar, V.; Choudhry, V.P. Iron deficiency and infection. Indian J. Pediatr. 2010, 77, 789–793.
- Litton, E.; Lim, J. Iron Metabolism: An Emerging Therapeutic Target in Critical Illness. Crit. Care 2019, 23, 81.
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276.
- Hippchen, T.; Altamura, S.; Muckenthaler, M.U.; Merle, U. Hypoferremia is Associated with Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere 2020, 10, e492.
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48.
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555.
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be Well: A Potential Role for Vitamin B in COVID-19. Maturitas 2021, 144, 108–111.
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R. Pathogen Reduction of SARS-CoV-2 Virus in Plasma and Whole Blood Using Riboflavin and UV Light. PLoS ONE 2020, 15, e0233947.
- Beigmohammadi, M.T.; Bitarafan, S.; Hoseindokht, A.; Abdollahi, A.; Amoozadeh, L.; Soltani, D. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: A randomized clinical trial. Trials 2021, 22, 802.
- Wiltshire, E.; Peña, A.S.; MacKenzie, K.; Shaw, G.; Couper, J. High dose folic acid is a potential treatment for pulmonary hypertension, including when associated with COVID-19 pneumonia. Med. Hypotheses 2020, 143, 110142.
- Ibrahimagić, O.Ć.; Kunić, S. Comment on an article: “High dose folic acid is a potential treatment for pulmonary hypertension, including when associated with COVID-19 pneumonia”. Med. Hypotheses 2020, 145, 110338.
- Narayanan, N.; Nair, D.T. Vitamin b12 May Inhibit Rna-Dependent-Rna Polymerase Activity of NSP12 from the SARS-CoV-2 Virus. IUBMB Life 2020, 72, 2112–2120.
- Tengelmann, C.; Joos, S.; Kaußner, Y.; Malzahn, U.; Lunden, L.; Klug, A.; Häusler, K.G.; Escales, C.; Maetzler, W.; Hügen, K.; et al. Feasibility, safety and effectiveness of prednisolone and vitamin B1, B6, and B12 in patients with post-COVID-19-syndrome (PreVitaCOV)—Protocol of a randomised, double-blind, placebo-controlled multicentre trial in primary care (phase IIIb). BMC Infect. Dis. 2024, 24, 56.
- Shahbaz, U.; Fatima, N.; Basharat, S.; Bibi, A.; Yu, X.; Hussain, M.I.; Nasrullah, M. Role of vitamin C in preventing of COVID-19 infection, progression and severity. AIMS Microbiol. 2022, 8, 108–124.
- Tehrani, S.; Yadegarynia, D.; Abrishami, A.; Moradi, H.; Gharaei, B.; Rauofi, M.; Nejad, F.M.; Sali, S.; Khabiri, N.; Abolghasemi, S. An Investigation into the Effects of Intravenous Vitamin c on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia a Randomized Clinical Trial. Urol. J. 2021, 19, 460–465.
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of L-Arginine plus Vitamin c Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984.
- Jamali Moghadam Siahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and Effectiveness of High-Dose Vitamin c in Patients with COVID-19: A Randomized Open-Label Clinical Trial. Eur. J. Med. Res. 2021, 26, 20.
- Fogleman, C.; Cohen, D.; Mercier, A.; Farrell, D.; Rutz, J.; Bresz, K.; Vernon, T. A Pilot of a Randomized Control Trial of Melatonin and Vitamin c for Mild-To-Moderate COVID-19. J. Am. Board Fam. Med. 2022, 35, 695–707.
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157.
- Al Sulaiman, K.; Aljuhani, O.; Saleh, K.B.; Badreldin, H.A.; Al Harthi, A.; Alenazi, M.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Alhammad, A.M.; et al. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: A propensity score matched study. Sci. Rep. 2021, 11, 17648.
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988.
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E. COVIT-TRIAL study group. COVID-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1031.
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170.
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; The, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017.
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients 2021, 13, 1714.
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, C.; Coussens, A.K.; Richter, A.G.; et al. Vitamin D Supplementation Does Not Influence SARS-CoV-2 Vaccine Efficacy or Immunogenicity: Sub-Studies Nested within the CORONAVIT Randomised Controlled Trial. Nutrients 2022, 14, 3821.
- Nicoll, R.; Henein, M.Y. COVID-19 Prevention: Vitamin D Is Still a Valid Remedy. J. Clin. Med. 2022, 11, 6818.
- Romero-Ibarguengoitia, M.E.; Gutiérrez-González, D.; Cantú-López, C.; Sanz-Sánchez, M.Á.; González-Cantú, A. Effect of Vitamin D3 Supplementation vs. Dietary-Hygienic Measures on SARS-CoV-2 Infection Rates in Hospital Workers with 25-Hydroxyvitamin D3 Levels ≥20 ng/mL. Microorganisms 2023, 11, 282.
- D’Ecclesiis, O.; Gavioli, C.; Martinoli, C.; Raimondi, S.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Palorini, R.; et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268396.
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of N-3 Pufas: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306.
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125.
- Lakshimi, V.I.; Kavitha, M. New Insights into Prospective Health Potential of ω-3 PUFAs. Curr. Nutr. Rep. 2023, 12, 813–829.
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashemi, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; et al. Omega-3 polyunsaturated fatty acid supplementation improve clinical symptoms in patients with 64 COVID-19: A randomized clinical trial. Int. J. Clin. Pract. 2021, 75, e14854.
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146.
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128.
- Zárate, R.; Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017, 6, 25.
- Mohajeri, M.; Horriatkhah, E.; Mohajery, R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: A case–control study. Inflammopharmacology 2021, 29, 1769–1776.
- de Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Hoebers, V.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411.
- Nicodemo, M.; Spreghini, M.R.; Manco, M.; Wietrzykowska Sforza, R.; Morino, G. Childhood Obesity and COVID-19 Lockdown: Remarks on Eating Habits of Patients Enrolled in a Food-Education Program. Nutrients 2021, 13, 383.
- Rodriguez, J.A.M.; Bifano, M.; Roca Goma, E.; Plasencia, C.M.; Torralba, A.O.; Font, M.S.; Millán, P.R. Effect and Tolerability of a Nutritional Supplement Based on a Synergistic Combination of β-Glucans and Selenium-and Zinc-Enriched Saccharomyces cerevisiae (ABB C1®) in Volunteers Receiving the Influenza or the COVID-19 Vaccine: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 4347.
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, A.T.Y.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in COVID-19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899.
- Brahma, S.; Naik, A.; Lordan, R. Probiotics: A gut response to the COVID-19 pandemic but what does the evidence show? Clin. Nutr. ESPEN 2022, 51, 17–27.
- Su, Q.; Liu, Q.; Zhang, L.; Xu, Z.; Liu, C.; Lu, W.; Ching, J.Y.L.; Li, A.; Mak, J.W.Y.; Lui, G.C.Y.; et al. Antibiotics and probiotics impact gut antimicrobial resistance gene reservoir in COVID-19 patients. Gut Microbes 2022, 14, 2128603.
- Rathi, A.; Jadhav, S.B.; Shah, N. A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue. Medicines 2021, 8, 47.
More
