Neurodegenerative diseases and Alzheimer’s disease (AD), as one of the most common causes of dementia, result in progressive losses of cholinergic neurons and a reduction in the presynaptic markers of the cholinergic system. These consequences can be compensated by the inhibition of acetylcholinesterase (AChE) followed by a decrease in the rate of acetylcholine hydrolysis. The assessment of cholinesterase inhibitors includes the comparison of the biosensor signals recorded prior to and after the contact of the enzyme with an inhibitor. This makes it possible to calculate the inhibition degree as the relative shift of the enzyme activity and as a measure of the inhibitor content. Even in the case of the direct signal measurement performed in the presence of both the substrate and inhibitor, it is mostly assumed that the initial enzyme activity (for zero inhibitor concentration) is constant and reproducible in the series of experiments required for calibration graph plotting.
- electrochemical biosensor
- acetylcholinesterase
- reversible inhibition
- drug determination
1. Cholinesterase Immobilization
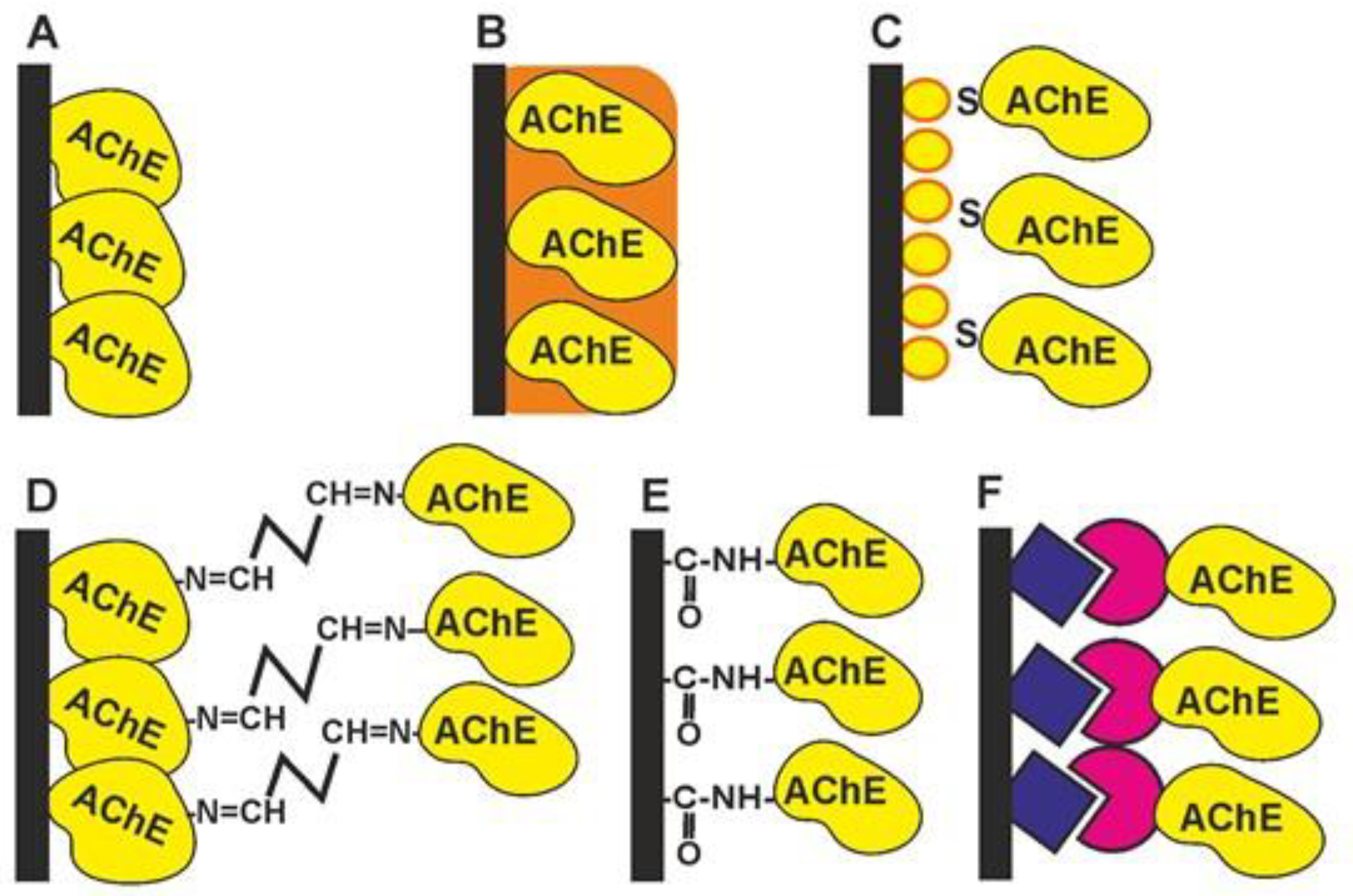
-
Physical adsorption on solid support (Figure 41A). The bare and modified surface of the electrode as a primary signal transducer or plastic films attached to such an electrode can be used as enzyme solid supports. This method offers the high stability of the enzyme during the storage of the biosensor due to the hydrophilic microenvironment of the enzyme established in the surface layer. Physical adsorption on the solid support including entrapment in the polymer gels (Figure 41B) or in the polyelectrolyte complexes makes it possible to preserve the native structure of an enzyme globule and its affinity toward inhibitors. Polyurethane [56][1], polyaniline [57,[58,259]][3][4], polypyrrole [60][5], polysiloxanes [61][6], sodium alginate [62][7], poly(vinyl acetate) photocurable polymer (PVA-SbQ) [63][8] and bovine serum albumin (BSA) [64][9] were used for this purpose. Possible leaching (desorption) of the enzyme can be suppressed by additional cover films deposited or attached to the enzyme layer. A similar approach has been described for the simultaneous immobilization of AChE with an auxiliary enzyme, choline oxidase (ChO) [65][10]. Carbon nanomaterials offer many advantages as enzyme supports due to a high surface to volume ratio, electroconductivity and a high adsorption capacity [66,67][11][12].
-
The formation of self-assembled monolayers (Figure 41C) is specified because of the high importance of this immobilization protocol for biosensors utilizing golden electrodes or nanoparticles in their assembly [68,69,70,71][13][14][15][16]. The formation of Au-S bonds offers the site-specific surface immobilization of enzyme molecules. The use of thiolated linkers makes it possible to extend the surface layer and increase its accessibility for inhibitors. Au, as a highly conductive material, improves the conditions of electron transduction and enzyme electric “wiring” and is often combined with other modifiers added to increase the specific surface area (carbon nanomaterials, chitosan films, electropolymerized coatings, etc.).
-
Cross-linking with glutaraldehyde (Figure 41D) increases the average molar mass of the protein so that the enzyme becomes insoluble and deposits on the solid support [64,72][9][17]. Glutaraldehyde interacts with amino and thiol functional groups to form Schiff bases. Although the reaction is reversible, the reverse hydrolysis of the product is less probable in the biosensor operation period. The reaction is mostly performed in the presence of the BSA protein protecting the active site of the enzyme from undesired chemical reactions. The reaction is complicated by the partial oligomerization of glutaraldehyde during storage.
-
Covalent carbodiimide binding (Figure 41E) with carboxylated carriers [73,74][18][19]. Carbodiimide, specifically, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide chloride (EDC), forms amide bonds between the carboxylic and amino functions and provides the site-specific immobilization of proteins to carboxylated materials, e.g., carbon nanotubes or carbon black. The reaction is performed in mild conditions (at room temperature). The addition of N-hydroxysuccinimide (NHS) prevents the hydrolysis of the unstable intermediate and increases the efficiency of the enzyme binding. Carbodiimide binding is easily combined with the use of Au supports covered with monolayers of mercaptopropionic and mercaptoundecanoic acids.
2. Cholinesterase Biosensor Signal Measurement
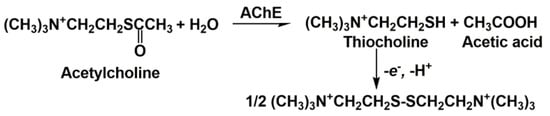
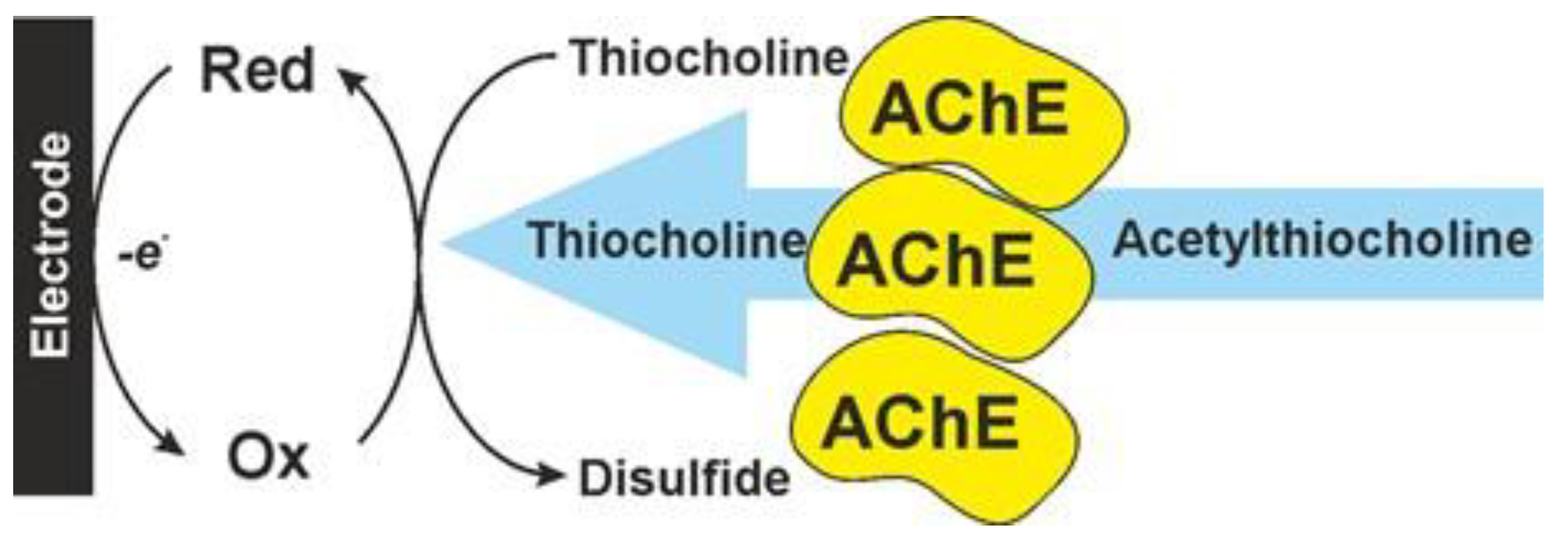
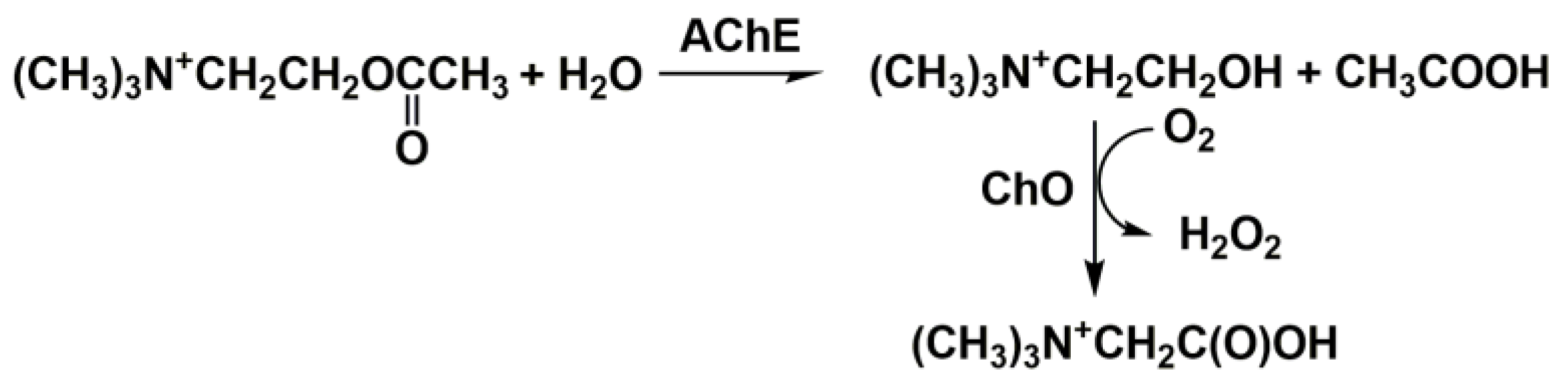
3. Anti-AD Drugs Determination
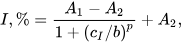
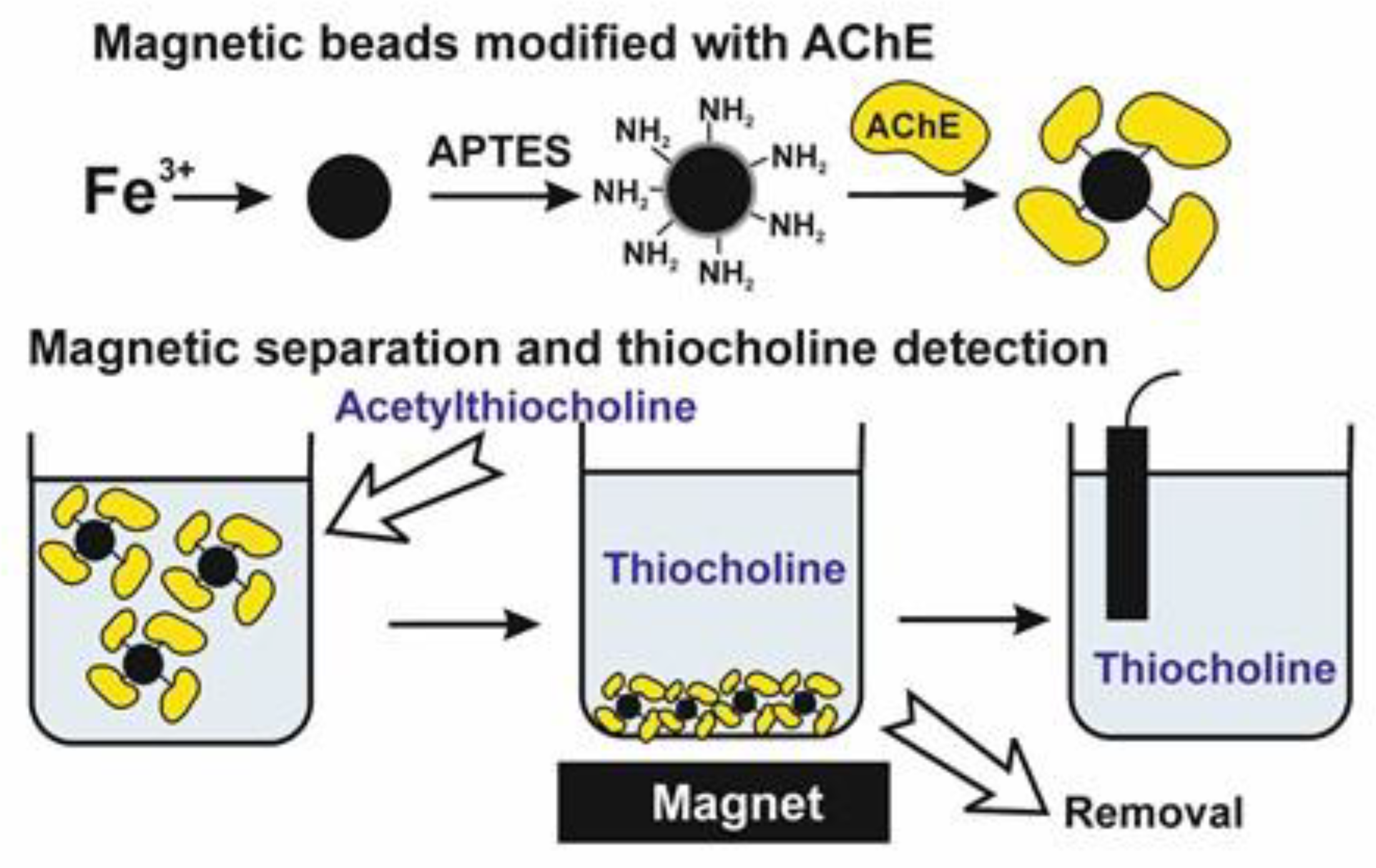
| Surface Layer Assembly | Signal Measurement | Inhibitor, Concentration Range, LOD | Ref. |
|---|---|---|---|
| GCE modified with CB, pillar [6]arene and nanoAg, AChE from electric eel covalently immobilized via carbodiimide binding | Amperometry, mediated thiocholine oxidation | LODs: huperzine A 1.2 nM, galantamine 12.5 nM, donepezil 2.5 nM, berberine 10 nM | [74][19] |
| GCE modified with CB and Co phtalocyanine, AChE from electric eel implemented in the PAA-PSS polyelectrolyte complexes | Amperometry, mediated oxidation of thiocholine | Donepezil, 0.1 nM–1.0 µM, IC50 24.7 nM Berberine 10 nM–0.1 mM, IC50 590 nM |
[84][29] |
| Carbon paste electrode with TTF-TCNQ/ionic liquid, AChE included in the ionic liquid gel | Amperometry, thiocholine oxidation | Eserine 0.1–1000 nM, LOD 0.026 nM | [85][30] |
| GCE modified with CB and pillar [6]arene, AChE from electric eel covalently immobilized via carbodiimide binding | Amperometry, mediated thiocholine oxidation | Berberine 10 nM–10 µM, LOD 1.0 nM | [87][32] |
| Au electrode modified with PAMAM dendrimer and co-immobilized AChE from electric eel and ChO via Au-S binding (amperometry) or adsorbed on polyaniline (potentiometry) | Amperometry, H2O2 oxidation, potentiometry, polyaniline equilibrium potential shift | Eserine, LOD 0.1 and 7000 nM for amperometric and potentiometric sensors, respectively | [97][42] |
| Screen-printed Au electrode | DPV, thiocholine oxidation, AChE in solution | Donepezil, IC50 28 ± 7 nM | [101][46] |
| GCE/rGO covered with L-cysteine–Ag(I) coordination polymer in Nafion matrix | Amperometry, H2O2 reduction in the mixture of AChE–ChO incubated with acetylthiocholine and donepezil | Donepezil, IC50 1.4 nM Tacrine IC50 3.5 nM |
[102][47] |
| SPCE with poly(methacrylate) and physically adsorbed AChE from electric eel | Amperometry, thiocholine oxidation | Eserine, 3–1000 ng/mL, IC75 2 µg/mL | [104][49] |
| Graphite electrode covered with electropolymerized thiophene derivative covalently attached to AChE and ChO | Amperometry | Donepezil, 0.4–5.0 and 5–50 µg/L, LOD 27 ng/L | [105][50] |
| Au disk electrode modified with cysteamine and AChE from electric eel covalently attached via Au-S bonding | Amperometry, thiocholine oxidation | IC50, µM: donepezil 0.50, neostigmine 0.71, eserine 0.77, tacrine 1.37, galantamine 1.20 | [106][51] |
| SPCE modified with CB and pillar [5]arene, the AChE from electric eel immobilized via carbodiimide binding to the inner walls of flow cell | Amperometry, mediated thiocholine oxidation | Donepezil 1.0 nM–1.0 µM, IC50 40 nM, LOD 0.5 nM. Berberine 1.0 µM–1.0 mM, IC50 1.24 µM, LOD 0.12 µM |
[107][52] |
| SPCB on paper support with Prussian blue and BChE from horse serum immobilized via spotting the filter paper | Amperometry, mediated thiocholine oxidation | Physostigmine: 0.01–1.0 µM, LOD 5 nM | [108][53] |
| GCE modified with nanoAu/silica gel and physically adsorbed AChE from electric eel | Amperometry, oxidation of thiocholine | Galantamine and neostigmine 0–10 µM | [110][55] |
| GCE/nanoPd/g-C3N4/adsorbed AChE | Amperometry, oxidation of thiocholine | Huperzine A 3.89 nM–20.80 µM, LOD 1.30 nM | [111][56] |
| SCE containing α-cyclodextrin in PVC matrix, AChE or BChE in solution incubated with an inhibitor | Potentiometry | Rivastigmine 0.247–1.45, LOD 0.097, Pyridostigmine 0.179–0.975, LOD 0.113, meclofenoxate 375–1725, LOD 30.924, memantine 3.55–18.38, LOD 1.056, methotrexate 1.0–37.0, LOD 3.557, cyclopentolate 2.88–15.6, LOD 0.947, oxfendazole 3.12–34.32, LOD 1.434, carbazepine 2.344–9.50, LOD 0.590 ng/mL | [112][57] |
References
- Hart, A.L.; Collier, W.A.; Janssen, D. The response of screen-printed enzyme electrodes containing cholinesterases to organo-phosphates in solution and from commercial formulations. Biosens. Bioelectron. 1997, 12, 645–654.
- Paneru, S.; Kumar, D. A Novel electrochemical biosensor based on polyaniline-embedded copper oxide nanoparticles for high-sensitive paraoxon-ethyl (PE) detection. Appl. Biochem. Biotechnol. 2023, 195, 4485–4502.
- Rachmawati, A.; Sanjaya, A.R.; Putri, Y.M.T.A.; Gunlazuardi, J.; Ivandini, T.A. An acetylcholinesterase-based biosensor for isoprocarb using a gold nanoparticles-polyaniline modified graphite pencil electrode. Anal. Sci. 2023, 39, 911–923.
- Shrestha, D.; Nayaju, T.; Shrestha, B.K.; Maharjan, B.; Kang, K.; Bacirhonde, P.M.; Park, C.H.; Kim, C.S. Fabrication of flexible glucose sensor based on heterostructure ZnO nanosheets decorated PU/Chitosan-PANI hybrid nanofiber. Microchem. J. 2024, 197, 109915.
- Loguercio, L.F.; Thesing, A.; Demingos, P.; de Albuquerque, C.D.L.; Rodrigues, R.S.B.; Brolo, A.G.; Santos, J.F.L. Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sens. Actuators B 2021, 339, 129875.
- Raghu, P.; Swamy, B.E.K.; Madhusudana, R.T.; Chandrashekar, B.N.; Reddaiah, K. Sol-gel immobilized biosensor for the detection of organophosphorous pesticides: A voltammetric method. Bioelectrochemistry 2012, 83, 19–34.
- Weng, Y.; Yang, Y.; Li, Y.; Xu, L.; Chen, X.; Song, H.; Zhao, C.-X. Alginate-based materials for enzyme encapsulation. Adv. Colloid Interface Sci. 2023, 318, 102957.
- Nunes, G.S.; Jeanty, G.; Marty, J.-L. Enzyme immobilization procedures on screen-printed electrodes used for the detection of anticholinesterase pesticides: Comparative study. Anal. Chim. Acta 2004, 523, 107–115.
- Kumar, T.H.V.; Sundramoort, A.K. Electrochemical biosensor for methyl parathion based on single-walled carbon nanotube/glutaraldehyde crosslinked acetylcholinesterase-wrapped bovine serum albumin nanocomposites. Anal. Chim. Acta 2019, 1074, 131–141.
- López, M.S.-P.; Pérez, J.P.H.; López-Cabarcos, E.; López-Ruiz, B. Amperometric biosensors based on choline oxidase entrapped in polyacrylamide microgel. Electroanalysis 2007, 19, 370–378.
- Qin, Y.; Jiang, X.; Wang, X.; Gao, X.; Zhao, L. Luminescent solid-state carbon dots synthesized based on the one-step hydrothermal method for the immobilization of α-glucosidase and imaging screening of enzyme inhibitors. J. Alloys Compd. 2024, 978, 173475.
- Kumar, A.; Purohit, B.; Mahato, K.; Roy, S.; Srivastava, A.; Chandra, P. Design and development of ultrafast sinapic acid sensor based on electrochemically nanotuned gold nanoparticles and solvothermally reduced graphene oxide. Electroanalysis 2020, 32, 59–69.
- Phongphut, A.; Chayasombat, B.; Cass, A.E.G.; Sirisuk, A.; Phisalaphong, M.; Prichanont, S.; Thanachayanont, C. Clay/Au nanoparticle composites as acetylcholinesterase carriers and modified-electrode materials: A comparative study. Appl. Clay Sci. 2020, 194, 105704.
- Vu, T.T.; Dau, T.N.N.; Ly, C.T.; Pham, D.C.; Nguyen, T.T.N.; Pham, V.T. Aqueous electrodeposition of (AuNPs/MWCNT–PEDOT) composite for high-affinity acetylcholinesterase electrochemical sensors. J. Mater. Sci. 2020, 55, 9070–9081.
- Hu, X.; Dinu, C.Z. Nanoporous gold electrode for ultrasensitive detection of neurotoxin fasciculin. Anal. Chim. Acta 2019, 1085, 91–97.
- Handali, P.R.; Webb, L.J. Gold nanoparticles are an immobilization platform for active and stable acetylcholinesterase: Demonstration of a general surface protein functionalization strategy. ACS Appl. Bio Mater. 2023, 6, 209–217.
- Theyagarajan, K.; Kim, Y.-J. Recent developments in the design and fabrication of electrochemical biosensors using functional materials and molecules. Biosensors 2023, 13, 424.
- Khaldi, K.; Sam, S.; Lounas, A.; Yaddaden, C.; Gabouze, N.-E. Comparative investigation of two methods for acetylcholinesterase enzyme immobilization on modified porous silicon. Appl. Surf. Sci. 2017, 421A, 148–154.
- Shamagsumova, R.V.; Vasyk, A.V.; Shurpik, D.N.; Evtyugin, V.G.; Stoikov, I.I.; Evtyugin, G.A. An acetylcholinesterase sensor based on a pillararene–silver nanoparticle composite for the determination of drugs for the treatment of Alzheimer’s disease. J. Anal. Chem. 2022, 77, 429–438.
- Zhang, J.; Li, Y.; Zhang, T.; Zheng, Z.; Jing, H.; Liu, C. Improving pesticide residue detection: Immobilized enzyme microreactor embedded in microfluidic paper-based analytical devices. Food Chem. 2024, 439, 138179.
- Zhang, J.; Li, Y.; Chen, L.; Zheng, Z.; Liu, C. Screening of acetylcholinesterase inhibitors by capillary electrophoresis with oriented-immobilized enzyme microreactors based on gold nanoparticles. Molecules 2024, 29, 118.
- Rajagopalan, V.; Venkataraman, S.; Rajendran, D.S.; Kumar, V.V.; Kumar, V.V.; Rangasamy, G. Acetylcholinesterase biosensors for electrochemical detection of neurotoxic pesticides and acetylcholine neurotransmitter: A literature review. Environ. Res. 2023, 227, 115724.
- Zhang, P.; Sun, T.; Rong, S.; Zeng, D.; Yu, H.; Zhang, Z.; Chang, D.; Pan, H. A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH2 nanocomposite. Bioelectrochemistry 2019, 127, 163–170.
- Song, D.; Wang, Y.; Lu, X.; Gao, Y.; Li, Y.; Gao, F. Ag nanoparticles-decorated nitrogen-fluorine co-doped monolayer MoS2 nanosheet for highly sensitive electrochemical sensing of organophosphorus pesticides. Sens. Actuators B 2018, 267, 5–13.
- Shamagsumova, R.V.; Efimova, O.Y.; Gorbatchuk, V.V.; Evtugyn, V.G.; Stoikov, I.I.; Evtugyn, G.A. Electrochemical acetylcholinesterase biosensor based on polylactide–nanosilver composite for the determination of anti-dementia drugs. Anal. Lett. 2019, 52, 1558–1578.
- Zhang, J.; Hu, H.; Yang, L. Ultra-highly sensitive and stable acetylcholinesterase biosensor based on TiO2-NRs and rGO. Microchem. J. 2021, 168, 106435.
- Zhao, C.; Zhao, Y.; Wang, L.; Lu, K.; Guo, W.; Lu, X.; Gao, F. AuNPs and CNTs embellished three-dimensional bloom-like α-Fe2O3 nanocomposites for highly sensitive electrochemical pesticides detection. Microchem. J. 2023, 191, 108762.
- Li, R.; Zhang, W.; Meng, F.; Li, X.; Li, Z.; Fang, Y.; Zhang, M. Hollow Prussian blue with ultrafine silver nanoparticle agents (Ag-HPB) integrated sensitive and flexible biosensing platform with highly enzyme loading capability. Talanta 2024, 266 Pt 1, 125036.
- Ivanov, A.; Davletshina, R.; Sharafieva, I.; Evtugyn, G. Electrochemical biosensor based on polyelectrolyte complexes for the determination of reversible inhibitors of acetylcholinesterase. Talanta 2019, 194, 723–730.
- Zamfir, L.-G.; Rotariu, L.; Bala, C. Acetylcholinesterase biosensor for carbamate drugs based on tetrathiafulvalene-tetracyanoquinodimethane / ionic liquid conductive gels. Biosens. Bioelectron. 2013, 46, 61–67.
- Shamagsumova, R.V.; Shurpik, D.N.; Evtugyn, V.G.; Stoikov, I.I.; Evtugyn, G.A. Electrochemical determination of malathion on an acetylcholinesterase-modified glassy carbon electrode. Anal. Lett. 2018, 51, 1911–1926.
- Shamagsumova, R.V.; Shurpik, D.N.; Kuzin, Y.I.; Stoikov, I.I.; Rogov, A.M.; Evtugyn, G.A. Pillararene: Electrochemistry and application in electrochemical (bio)sensors. J. Electroanal. Chem. 2022, 913, 116281.
- Wu, F.; Wang, B.; Guo, H.; Kang, K.; Ji, X.; Wang, L.; Guo, S.; Ren, J. Rational design of a novel MOF-based ternary nanocomposite for effectively monitoring harmful organophosphates in foods and the environment. Anal. Methods 2023, 15, 1168–1177.
- Hao, Y.; Zuo, X.; Lu, X.; Li, Z.; Gao, F. Hierarchical porous hollow N-doped Cu-based MOF derivatives as highly sensitive electrochemical sensing platform for pesticides detection. Sens. Actuators B 2022, 362, 131749.
- Jing, C.; Kuang, Y.; Gu, X.; Xu, M.; Wu, Y.; Wang, X. An acetylcholine electrochemical biosensor based on bi-enzyme functionalized nanofiber composites. J. Electrochem. Soc. 2023, 170, 077513.
- Upadhyay, S.; Rao, G.R.; Sharma, M.K.; Bhattacharya, B.K.; Rao, V.K.; Vijayaraghavan, R. Immobilization of acetylcholinesterase-choline oxidase on a gold-platinum bimetallic nanoparticles modified glassy carbon electrode for the sensitive detection of organophosphate pesticides, carbamates and nerve agents. Biosens. Bioelectron. 2009, 25, 832–838.
- Chauhan, N.; Balayan, S.; Jain, U. Sensitive biosensing of neurotransmitter: 2D material wrapped nanotubes and MnO2 composites for the detection of acetylcholine. Synth. Met. 2020, 263, 116354.
- Pchelintsev, N.A.; Millner, P.A. A novel procedure for rapid surface functionalisation and mediator loading of screen-printed carbon electrodes. Anal. Chim. Acta 2008, 612, 190–197.
- Kaniewska, M.; Jońca, J.; Połeć, I.; Sikora, T.; Marty, L.-L.; Trojanowicz, M. Enantioselective inhibition of immobilized acetylcholinesterase in biosensor determination of pesticides. Cent. Eur. J. Chem. 2012, 10, 1760–1765.
- Zheng, Z.; Li, X.; Dai, Z.; Liu, S.; Tang, Z. Detection of mixed organophosphorus pesticides in real samples using quantum dots/bi-enzyme assembly multilayers. J. Mater. Chem. 2011, 21, 16955–16962.
- Cao, X.; Guo, Y.; Zhao, M.; Li, J.; Wang, C.; Xia, J.; Zou, T.; Wang, Z. An efficient multi-enzyme cascade platform based on mesoporous metal-organic frameworks for the detection of organophosphorus and glucose. Food Chem. 2022, 381, 132282.
- Snejdarkova, M.; Svobodova, L.; Evtugyn, G.; Budnikov, H.; Karyakin, A.; Nikolelis, D.P.; Hianik, T. Acetylcholinesterase sensors based on gold electrodes modified with dendrimer and polyaniline. A comparative research. Anal. Chim. Acta 2004, 514, 79–88.
- Cuartero, M.; Pérez, S.; García, M.S.; García-Cánovas, F.; Ortuño, J.A. Comparative enzymatic studies using ion-selective electrodes. The case of cholinesterases. Talanta 2018, 180, 316–322.
- Cuartero, M.; García, M.S.; García-Cánovas, F.; Ortuño, J.Á. New approach for the potentiometric-enzymatic assay of reversible-competitive enzyme inhibitors. Application to acetylcholinesterase inhibitor galantamine and its determination in pharmaceuticals and human urine. Talanta 2013, 110, 8–14.
- Ghindilis, A.L.; Morzunova, T.G.; Barmin, A.V.; Kurochkin, I.N. Potentiometric biosensors for cholinesterase inhibitor analysis based on mediatorless bioelectrocatalysis. Biosens. Bioelectron. 1996, 11, 873–880.
- Veloso, A.J.; Nagy, P.M.; Zhang, B.; Dhar, D.; Liang, A.; Ibrahim, T.; Mikhaylichenko, S.; Aubert, I.; Kerman, K. Miniaturized electrochemical system for cholinesterase inhibitor detection. Anal. Chim. Acta 2013, 774, 73–78.
- Zhang, Q.; Hu, Y.; Wu, D.; Ma, S.; Wang, J.; Rao, J.; Xu, L.; Xu, H.; Shao, H.; Guo, Z.; et al. Protein-mimicking nanowire-inspired electro-catalytic biosensor for probing acetylcholinesterase activity and its inhibitors. Talanta 2018, 183, 258–267.
- Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Zhang, F.; Wu, C. Droplet-based microfluidics for dose–response assay of enzyme inhibitors by electrochemical method. Anal. Chim. Acta 2013, 796, 68–74.
- Levien, M.; Farka, Z.; Pastucha, M.; Skladal, P.; Nasri, Z.; Weltmann, K.-D.; Fricke, K. Functional plasma-polymerized hydrogel coatings for electrochemical biosensing. Appl. Surf. Sci. 2022, 584, 152511.
- Turan, J.; Kesik, M.; Soylemez, S.; Goker, S.; Kolb, M.; Bahadir, M.; Toppare, L. Development of an amperometric biosensor based on a novel conducting copolymer for detection of anti-dementia drugs. J. Electroanal. Chem. 2014, 735, 43–50.
- Vandeput, M.; Parsajoo, C.; Vanheuverzwijn, J.; Patris, S.; Yardim, Y.; le Jeune, A.; Sarakbi, A.; Mertens, D.; Kauffmann, J.M. Flow-through enzyme immobilized amperometric detector for the rapid screening of acetylcholinesterase inhibitors by flow injection analysis. J. Pharm. Biomed. Anal. 2015, 102, 267–275.
- Ivanov, A.; Stoikov, D.; Shafigullina, I.; Shurpik, D.; Stoikov, I.; Evtugyn, G. Flow-through acetylcholinesterase sensor with replaceable enzyme reactor. Biosensors 2022, 12, 676.
- Caratelli, V.; Ciampaglia, A.; Guiducci, J.; Sancesario, G.; Moscone, D.; Arduini, F. Precision medicine in Alzheimer’s disease: An origami paper-based electrochemical device for cholinesterase inhibitors. Biosens. Bioelectron. 2020, 165, 112411.
- Kostelnik, A.; Kopel, P.; Cegan, A.; Pohanka, M. Construction of an acetylcholinesterase sensor based on synthesized paramagnetic nanoparticles, a simple tool for neurotoxic compounds assay. Sensors 2017, 17, 676.
- Du, D.; Chen, S.; Cai, J.; Song, D. Comparison of drug sensitivity using acetylcholinesterase biosensor based on nanoparticles-chitosan sol-gel composite. J. Electroanal. Chem. 2007, 611, 60–66.
- Wang, B.; Ye, C.; Zhong, X. Electrochemical biosensor for organophosphate pesticides and Huperzine-A detection based on pd wormlike nanochains/graphitic carbon nitride nanocomposites and acetylcholinesterase. Electroanalysis 2016, 28, 304–311.
- Omran, S.; Shoukry, E.M.; Mohamed, E.F.; Khaled, E.; El-Attar, R.O. Novel simple enzymatic potentiometric approach for toxicological assessment of anticholinesterase and Alzheimer’s drugs Enzymatic approach toxicological assessment. Egypt. Pharm. J. 2022, 21, 472–481.
