You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by José Alberto Peña Flores Portillo.
Extracellular vesicles (EVs) are defined as subcellular structures limited by a bilayer lipid membrane that function as important intercellular communication by transporting active biomolecules, such as proteins, amino acids, metabolites, and nucleic acids, including long non-coding RNAs (lncRNAs). These cargos can effectively be delivered to target cells and induce a highly variable response. LncRNAs are functional RNAs composed of at least 200 nucleotides that do not code for proteins. The angiogenesis process is fundamental for cancer to advance locally and facilitate metastasis, and therefore, it has been extensively studied in most cancer types.
- exosomes
- lncRNAs
- long non-coding RNAs
- circRNAs
- cancer
- angiogenesis
1. Introduction
1.1. Cancer Generalities
Cancer is defined as a group of diseases that are multifactorial in nature and represent a challenge in their diagnosis and treatment due to their etiological diversity [1]. More than 200 types of human cancer have been identified based on the cell or tissue from where they originate, the somatic mutations acquired at any time of the progression of the disease, and the microenvironment influences in which they develop [2]. One of the hallmark features of cancer is its rapid and uncontrolled progression due to mutations that alter the cell cycle and overpass checkpoint regulation between the cell cycle phases, promoting the accumulation of mutations passed down to the progeny [3]. For this rapid progression to occur, the growing tumor has a high demand for nutrients and other components; thus, these cells generate molecular signaling to promote the formation of new blood vessels from preexisting ones, a process denominated angiogenesis [4]. The angiogenesis process is fundamental for cancer to advance locally and facilitate metastasis, and therefore, it has been extensively studied in most cancer types [5,6,7][5][6][7]. Multiple efforts have been made to develop antiangiogenic therapies to halt tumor growth and prevent metastasis [8,9][8][9]. Recently, the role of extracellular vesicles (EVs) between vessels in tumor communication has triggered the interest of many researchers.
1.2. Extracellular Vesicles
Extracellular vesicles are subcellular structures that are heterogeneous in nature and surrounded by a lipid bilayer membrane that exerts multiple functions in intercellular communication [10]. Based on how they are delivered from the original cell to the extracellular medium, EVs can be released by inward budding of the endosomal membrane or outward budding of the cellular membrane [11]. The recipient cell can then internalize EVs through endocytosis or membrane fusion to unload their contents into the cell cytoplasm (Figure 1) [12]. Since their discovery in the early 1980s, many biomolecules have been identified as cargo in EVs, including proteins, amino acids, signaling lipids, and different genetic molecules like DNA, RNA, and non-coding RNAs, promoting both physiological and pathological processes [13]. Based on their biogenesis, EVs are generally classified into microvesicles and exosomes [10], although some authors suggest a further division into apoptotic bodies and proteasomes [14]. Microvesicles are generated by outward budding of the plasma membrane and range from 50 nm to 1000 nm. In contrast, exosomes are membrane vesicles smaller in size (30–100 nm) and are formed by inward budding of the endosomal membrane to be later secreted by fusion with the cell membrane [14,15][14][15]. The role of EVs as cell-to-cell mediators in respiratory disease [16], neurodegenerative disease [17], kidney disease [18], cardiovascular disease [19], and cancer progression and metastasis [20,21,22][20][21][22] has been documented to ameliorate the understanding of the behavior or these subcellular structures.
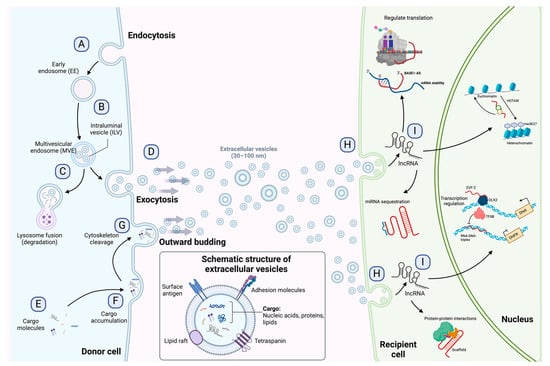
Figure 1. Exosome biogenesis, release to the extracellular environment, and uptake by the recipient cell. (A) Exosome biogenesis begins with early endosome formation during endocytosis. (B) Early endosomes are then matured into late endosomes, generating multiple intraluminal vesicles (ILVs) by the inward budding of endosomal membranes. (C) The accumulation of ILVs leads to the formation of multivesicular endosomes (MVEs), and proteins and nucleic acids produced by the donor cell can be sorted into exosomes during MVE formation. (D) Exosomes are released into the extracellular environment by fusing MVEs with the cellular membrane. (E–G) Microvesicles arise from the outward budding and shedding of the plasma membrane. (H) Extracellular vesicles are taken up by the recipient cell by direct fusion, receptor-mediated fusion, or endocytosis. (I) Exosomal lncRNAs can be subsequently delivered to the recipient cell to exert regulatory effects as sponges for miRNAs, protein scaffolds, transcription and translation regulators, and chromatin activators. The detailed functions of lncRNAs are depicted in Figure 2. Figure 1 is modified from Wang et al. [23].
1.3. Long Non-Coding and circRNAs
Long non-coding RNAs (lncRNAs) are a diverse group of RNAs that are not translated into proteins, and they are at least 200 nucleotides in length [24]. Recent advances in genomic sciences through RNA sequencing have offered the identification of lncRNAs performing functions to control chromatin complexes, recruit transcription factors, regulate alternative splicing, affect mRNA translation, sponge micro-RNAs by binding, degrade other RNAs, and serve as scaffolds for protein interactions (Figure 2) [25,26,27][25][26][27]. Evidence suggests an active role of lncRNAs in most physiological processes, and their involvement in disease has been the focus of active research in recent years [28,29][28][29]. The involvement of lncRNAs as oncogenes or tumor suppressors in many cancer types has also been documented. However, as new lncRNAs are discovered, the general landscape becomes complicated as the roles they can perform become more complex [30]. For instance, lncRNAs can influence the progression of cancer by promoting metastasis [31], drug resistance [32], epithelial-to-mesenchymal transition (EMT) [33], and angiogenesis [34].
It has recently been shown that some lncRNAs can take a circular shape and join covalently at the ends, and these are called circRNAs [35]. This type of lncRNA can perform similar functions to linear lncRNAs, as sponges to recruit specific miRNAs or as effectors to regulate the expression of certain genes [36]. circRNAs have recently been widely studied, arousing interest due to their stability, since, unlike linear non-coding RNAs, they are difficult to degrade [37].
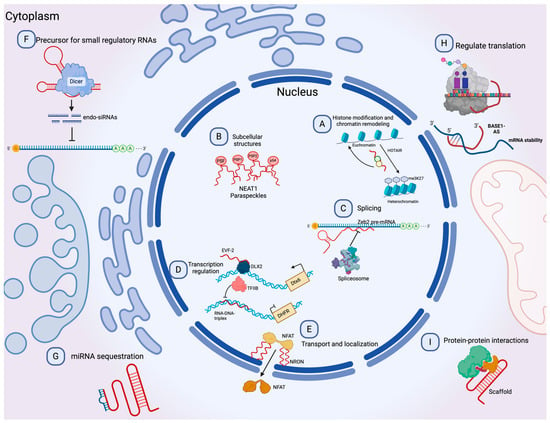
Figure 2. Molecular functions of lncRNAs. (A) lncRNAs can guide chromatin complexes, controlling between transcriptionally active euchromatin and silent heterochromatin. (B) The recruitment of polymerase II and transcription factors can be inhibited or facilitated by lncRNAs. (C) lncRNAs contribute to transcriptome complexity by regulating alternative splicing of pre-mRNAs. (D) lncRNAs affect the stability and translation of mRNA by base-pairing with mRNA molecules. (E) They influence the expression of miRNAs by binding to them and preventing their function. (F) lncRNAs can act as siRNAs and target other RNAs, which subsequently could result in target degradation. (G) lncRNAs can join multiple protein factors as flexible scaffolds to interact with or cooperate in protein–protein interactions. (H,I) The scaffold function is also important for protein activity and localization as well as subcellular structures. Modified from Peña-Flores et al. [38].
Recently, the presence of both coding and non-coding RNA in EVs has motivated research to elucidate RNA’s role in various biological mechanisms in cancer and other diseases [39,40][39][40].
2. Mechanism of Angiogenesis
The angiogenesis process embodies forming new blood vessels from existing vessels in response to physiological and pathological mechanisms [41]. During embryogenesis, the vascular network develops through a combination of vasculogenesis, referred to as the de novo formation of the heart and new blood vessels from stem endothelial cells, namely, angioblasts, and angiogenesis, which expands the initial primitive vascular plexus [42]. Although most blood vessels remain quiescent under physiological conditions, tissue repair and regeneration through wound healing, ovulation, and endometrial thickening throughout the menstrual cycle are based on angiogenesis for proper functioning [43,44,45][43][44][45]. While vascular growth varies depending on where angiogenesis is initiated and the tissue to which they will provide a new blood supply, several mechanisms are common in forming these vessels [46]. In a hypoxic state, the recruitment of cells that promote inflammation; angiogenic growth factor production; degradation of the basement membrane; and endothelial cells (ECs) sprouting, migrating, proliferating, differentiating, and modulating vascular support cells are some of the shared characteristics in angiogenesis [47]. The angiogenic process (Figure 3) comprises several stages involving the sprouting, migration, and proliferation of ECs guided by the vascular endothelial growth factor (VEGF) [48]. Following VEGF stimulation, pericytes from the vessel wall detach, and the basal membrane is weakened by proteolytic degradation. At the same time, ECs adopt an invasive and motile phenotype called tip cells that send out filamentous pseudopodia to guide vascular budding [49]. The cells behind the tip cells are denominated stalk cells, which proliferate to maintain the integrity of the structure and function of the nascent vessels, mainly expanding the vascular lumen [50]. ECs modify their shape by negatively charging glycoproteins on the apical surface to repel each other and open the lumen while redistributing cell-to-cell adhesion to the periphery [51]. For maturation to occur, pericytes must be recruited by the platelet-derived growth factor subunit B (PDGF-B) and angiopoietin 1 (Ang1) signaling along with the strengthening and consolidation of the adhesion between ECs with junctional molecules such as VE-cadherin, while a basement membrane is deposited by tissue inhibitors of metalloproteinases (TIMPs) [52,53][52][53].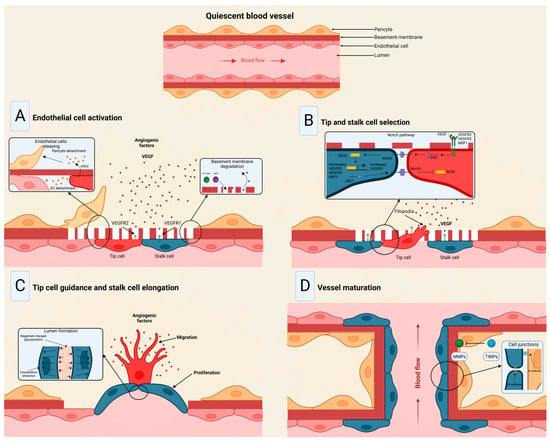
Figure 3. Stages of the angiogenic process. (A) Angiogenic signals, such as VEGF, promote pericyte detachment from the basement membrane and weaken the extracellular matrix. (B) Endothelial cells display characteristic phenotypes after VEGF stimulation: migratory tip cells or proliferating stalk cells. (C) Attractive and repulsive forces control endothelial cells, forming a vessel lumen to initiate blood flow. (D) PDGF-B and Ang1 signaling lead to pericyte recruitment, while junctional molecules consolidate EC–EC adhesion. Modified from Viallard et al. [47].
3. Exosomal Long Non-Coding and Circular RNAs in Cancer Angiogenesis
LncRNAs in EV cargo have been demonstrated lately, mainly in cancer [72,73,74][72][73][74]. A recent study launched an online repository of EV long RNAs (exLRs) in diverse human body fluids, comprising 19,643 mRNAs, 15,645 lncRNAs, and 79,084 circRNAs obtained from human blood, cerebrospinal fluid, bile, and urine samples. The database provides novel exLR signatures to help discover new biomarkers that could aid in diagnosing and treating many diseases [75]. Based on available recent research, Casado-Díaz et al. [76] concluded that lncRNAs and other RNAs included in MSC-derived EVs can be applied in chronic skin ulcers to improve accelerated healing and decrease scar formation due to immunosuppressive and immunomodulatory properties. Conversely, in a diabetic wound-healing animal model, upregulated lncRNAs packed in EVs from fibroblasts enhanced keratinocyte MMP-9 expression to induce collagen degradation, delaying wound healing [77]. Recently, the long non-coding repressor of NFAT (NRON) was detected in BMSC-derived EVs, inhibiting osteoclast differentiation and osteoporotic bone loss in vitro and in vivo [78]. In tumors, the high rate of cell proliferation forces the formation of new blood vessels [79]. However, in most cases, these blood vessels are dilated, tortuous, and immature, leading to excessive permeability and increased hypoxia [80]. In addition, vascular disorganization causes heterogeneity in the tumor blood vessel network, creating highly vascularized tumor areas and other hypoxic areas with low vascular density [47]. Thus, hypoxia becomes a major driver of tumor angiogenesis, along with other mechanisms promoted by activated oncogenes or loss of tumor suppressor genes, in which lncRNAs play an important role, mainly through acting as competing endogenous RNAs for miRNAs [81]. Similarly, circRNAs have been extensively studied in cancer, elucidating important roles in tumor development, growth, and angiogenesis [82]. For instance, VEGFR-related pathways have been linked to circRNAs by affecting tumor angiogenesis by sponging miRNAs [83,84][83][84]. The landscape of exosomal lnc- and circRNAs in angiogenesis in cancer is summarized in Table 1 and Figure 4.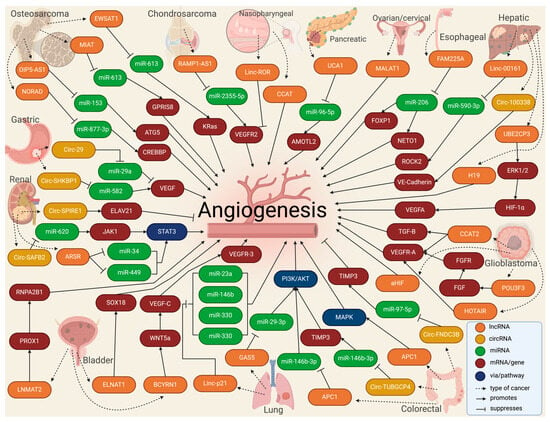
Figure 4.
Molecular landscape of exosomal lnc- and circRNAs in angiogenesis in cancer.
Table 1.
The landscape of exosomal lnc- and circRNAs in angiogenesis in cancer.
| Lnc/circRNA | Molecular Target | Donor Cells | Recipient Cells | Effect | Reference |
|---|---|---|---|---|---|
| OSTEOSARCOMA | |||||
| OIP5-AS1 | miR-153/ATG5 | HOS | HUVECs | Promotes | Li 2021 [85] |
| MIAT | miR-613/GPR158 | U2OS, MG63, and 293T | HUVECs | Promotes | Wang 2022 [86] |
| NORAD | miR-877-3p/CREBBP | 143B, MG-63, Saos2, HOS, and U20S | Osteosarcoma cells | Promotes | Feng 2022 [87] |
| EWSAT1 | miR-326/KRas | 143B, MNNG/HOS, MG63, U20S | BMSCs, HMEC-1 | Promotes | Tao 2020 [88] |
| CHONDROSARCOMA | |||||
| RAMP2-AS1 | miR-2355-5p/VEGFR2 | SW1353 | HUVECs | Promotes | Cheng 2020 [89] |
| PANCREATIC | |||||
| UCA1 | miR-96-5p/AMOTL2 | PANC-1, MIA PaCa-2, BxPC-3, Aspc-1, Sw1990 | HUVECs, HEK293T | Promotes | Guo 2020 [90] |
| NASOPHARYNGEAL | |||||
| Linc-ROR | p-AKT/p-VEGFR2 pathway | CNE2 | HUVECs | Promotes | Zhang 2022 [91] |
| CCAT2 | NR | CNE2, NP69 | HUVECs | Promotes | Zhou 2020 [92] |
| ESOPHAGEAL | |||||
| FAM225A | miR-206/NETO2 and FOXP1 | ECA109, TE-1, KYSE150, KYSE140 | HET-1A, HUVECs | Promotes | Zhang 2020 [93] |
| GLIOMA/GLIOBLASTOMA | |||||
| CCAT2 | VEGF-A and TGF-B | A172, U87-MG, U251, T98G | HUVECs | Promotes | Lang 2017 [94] |
| POU3F3 | bFGF/bFGFR/VEGFA | A172, U87-MG, U251, T98G | HBMECs | Promotes | Lang 2017 [95] |
| HOTAIR | VEGFA | A172 | HBMVECs | Promotes | Ma 2017 [96] |
| aHIF | NR * | U87-MG, U251-MG, A172, T98G | HUVECs | Promotes | Dai 2019 [97] |
| COLORECTAL | |||||
| CircFNDC3B | miR-97-5p/TIMP3 | LoVo, SW480, SW602, HCT116 | HUVECs | Suppresses | Zeng 2020 [98] |
| APC1 | MAPK pathway | HTC116, DLD-1, SW480, LoVo, SW116 | HEK293T, HUVECs | Promotes | Wang 2019 [99] |
| CircTUBGCP4 | miR-146b-3p/PDK2/Akt | SW480 | HEK297T | Promotes | Chen 2023 [100] |
| LIVER/HEPATOCELLULAR | |||||
| LINC00161 | miR-590-3p/ROCK2 axis | Huh-7, HCCLM3, MHCC-97L, MHCC-97H | WRL-68, HUVECs | Promotes | You 2021 [101] |
| UBE2CP3 | ERK1/2/HIF-1α/VEGFA | HepG2, SMMC-7721 | HUVECs | Promotes | Lin 2018 [102] |
| H19 | NR * | Huh-7, Sk-Hep | HUVECs | Promotes | Conigliaro 2015 [103] |
| Circ100338 | VE-Cadherin | Hep3B, HLE, Huh-7, BEL7402, SMCC7721, MHCC97L, HCCLM3, MHCC97H, HCCLM6 | HUVECs | Promotes | Huang 2020 [104] |
| LUNG | |||||
| MFI2-AS1 | miR-107/PI3K/AKT pathway | PC9, A549, H1299 | HUVECs | Promotes | Xu 2023 [105] |
| LincRNA-p21 | miR-23a, miR-146b, miR-330, and miR-494 | H23, HCC44 | HUVECs | Promotes | Castellano 2020 [106] |
| GAS5 | miR-29-3p/PI3K/Akt | 16HBE, A549, H1299, 95D | HUVECs | Promotes | Cheng 2019 [107] |
| RENAL CELL | |||||
| ARSR | miR-34 and miR-449 to upregulate STAT3 pathway | Caki-1, ACHN, 786-O | NR * | Promotes | Zheng 2022 [108] |
| CircSAFB2 | miR-620/JAK1/STAT3 axis | A498, 786-O, Caki-1, Caki-2, 769-P, ACHN | THP-1 | Promotes | Huang 2022 [109] |
| CircSPIRE1 | ELAVL1 protein | NR * | NR * | Suppresses | Shu 2023 [110] |
| BLADDER | |||||
| BCYRN1 | WNT5a/VEGF-C/VEGFR3 | T24, 5637, SVHUC-1 | HLECs, HDLECs, HUVECs | Promotes | Zheng 2021 [111] |
| LNMAT2 | PROX1/RNPA2B1/H3K4 | UM-UC-3, 5637, T24 | HLEC, SV-HUC-1 | Promotes | Chen 2020 [112] |
| ELNAT1 | SOX18 | UM-UC-1, RT112, RT4, UM-UC-3, T24, 5637 | HLEC, SV-HUC-1 | Promotes | Chen 2021 [113] |
| GASTRIC | |||||
| Circ0001190 | miR-587/SOSTDC1 | NR * | NR * | Suppresses | Liu 2022 [114] |
| Circ29 | miR-29a/VEGF pathway | SGC-7901, MGC-803 | HUVECs, HEK297T | Suppresses | Li 2021 [115] |
| CircSHKBP1 | miR-582/HUR/VEGF | AGS, HGC27, BGC823 MGC803, GES1 | HUVECs, HEK293T | Promotes | Xie 2020 [116] |
| CircFCHO2 | miR-194-5p/JAK1/STAT3 pathway | NR * | NR * | Promotes | Zhang 2022 [117] |
| OVARIAN | |||||
| MALAT1 | NR * | SKOV3, HO8910 | SKOV3.ip1, HO8910.PM | Promotes | Qiu 2018 [118] |
| CERVICAL | |||||
| TUG1 | VEGF-A, MMP-9, IL-8 | HeLa, CaSki | HUVECs | Suppresses | Lei 2020 [119] |
| BREAST | |||||
| CircHIPK3 | miR-124-3p/MTDH | NR * | NR * | Promotes | Shi 2022 [120] |
| THYROID | |||||
| FGD5-AS1 | miR-6838-5p/VAV2 axis | SW1736, KAT18 | HUVECs | Promotes | Liu 2022 [121] |
| MULTIPLE MIELOMA | |||||
| CircATP10A | miR-66758-3p, miR-3977, miR-6804-3p, miR-1266-3p, miR-3620-3p | NR * | NR * | Promotes | Yu 2022 [122] |
| ALCOHOL-INDUCED TUMOR | |||||
| HOTAIR and MALAT1 | NR * | NR * | HUVECs, HDMECs | Promotes | Lamichhane 2017 [123] |
| CHOLANGIOCARCINOMA | |||||
| CircCCAC1 | EZH2/SH3GL2 | CCA cells | HUVECs | Promotes | Xu 2021 [124] |
* NR: not reported.
3.1. Bone Malignancies
Some studies have been dedicated to studying exosomal lncRNAs in bone malignancies. LncRNA Opa-interacting protein 5-antisense 1 (OIP5-AS1) was found to be overexpressed in exosomes secreted by osteosarcoma cells, increasing angiogenesis in tubule formation assays by mechanistically sponging miR-153 and increasing the autophagy-related 5 protein (ATG5) [85]. Interestingly, serum samples from osteosarcoma patients could transfer via EVs the myocardial infarction-associated transcript (MIAT), promoting the proliferation of osteosarcoma cell lines and angiogenesis in HUVECs by sponging miR-613 and upregulating G protein-coupled receptor 158 (GPR158) [86]. In an in vitro and animal model, BMSC-EVs carried the non-coding RNA activated by DNA damage (NORAD) into osteosarcoma cells and upregulated CREB-binding protein (CREBBP) by sponging miR-877-3p to promote proliferation, invasion, migration, and angiogenesis [87]. Another lncRNA called Ewing sarcoma-associated transcript 1 (EWSAT1) was found to regulate osteosarcoma-induced angiogenesis via two mechanisms: (1) by increasing in sensitivity/reactivity of vascular endothelial cells triggered by exosomes carrying EWSAT1, and (2) by increasing angiogenic factors secretion [88]. Moreover, exosomes secreted by chondrosarcoma cells were loaded with the receptor activity-modifying protein 2 antisense 1 (RAMP2-AS1). They could enhance HUVECs proliferation, migration, and tube formation by acting as a ceRNA for miR2355-5p to regulate VEGFR2 expression. In addition, the overexpression of RAMP2-AS1 in the serum of chondrosarcoma patients was demonstrated to be closely related to local invasiveness, distant metastasis, and poor prognosis [89].3.2. Esophageal, Gastric, and Colorectal Cancers
Some of the most prevalent tumors of the gastrointestinal (GI) tract have been explored regarding the role played in angiogenesis by exosomes loaded with different lncRNAs and circRNAs. For instance, the exosomal lncRNA family with sequence similarity 225 member A (FAM225A) was highly expressed in esophageal squamous cell carcinoma (ESCC), upregulating neuropilin and tolloid-like 2 (NETO2) and forkhead box P1 (FOXP1) expression by sponging miR-206 to accelerate tumor progression and angiogenesis [93]. In gastric cancer patients, exosomal circ-SHKBP1 was overexpressed in tumor and blood samples. When the exosomes were isolated and exposed to different cell lines, cells showed a promoted proliferation, invasion, migration, and angiogenesis rate by mechanistically regulating the miR-582-3p/HUR/VEGF axis and suppressing heat shock protein 90 (HSP90) degradation [116]. Similarly, 30 blood samples and tissues from gastric cancer patients were taken to analyze circ-FCH and mu domain-containing endocytic adaptor 2 (FCHO2). It was found that circ-FCHO2 up-modulation led to a poor outcome, while circ-FCHO2 silencing weakened the proliferation, invasion, angiogenesis, and stem cell characteristics, presumably by activating the Janus kinase 1 (JAK1)/signal transducer and activator of transcription 2 (STAT2) pathway via sponging miR-194-5p [117]. Conversely, by acting as a miR-587 sponge to adjust the expression of the sclerostin domain-containing 1 (SOSTDC1), circ-0001190 overexpression inhibited cell viability, proliferation, angiogenesis, migration, and invasion of gastric cancer cell lines [114]. Moreover, circ-0044366 was highly expressed in gastric cancer and impaired the proliferation, migration, and tube formation of HUVECs by exosomal communication by acting as miR-29a ceRNA and regulating the VEGF pathway [115]. In colorectal cancer (CRC), tumor growth, angiogenesis, and liver metastasis were suppressed by exosomal circ-fibronectin type III domain-containing 3B (FNDC3B) overexpression by acting via the miR-97-5p/TIMP3 pathway [98]. Similarly, exosomes derived from lncRNA adenomatous polyposis coli (APC1)-silenced CRC cells promoted angiogenesis by activating the mitogen-activated protein kinase 1 (MAPK) pathway in endothelial cells, while enforced APC1 was sufficient to inhibit CRC growth, metastasis, and tumor angiogenesis by suppressing exosome production [99]. Interestingly, exosomes loaded with circ-tubulin gamma complex component 4 (TUBGCP4) derived from CRC cells enhanced vascular endothelial cell migration and tube formation via inducing filopodia formation and endothelial cell tipping by upregulating the pyruvate dehydrogenase kinase 2 (PDK2) to activate the AKT serine/threonine kinase 1 (AKT) signaling pathway and by sponging miR-146b-3p [100]. A very interesting study by Zhi et al. [125] compared EVs derived from the b-Raf proto-oncogene (BRAF) wild-type CRC and the BRAFV600E mutant patients to find the overexpression of 13 lncRNAs and downregulation of 22 lncRNAs in exosomes from the BRAFV600E mutation type. This difference showed a higher microvascular and micro-lymphatic vessel density of the BRAFV600E mutant CRC tissues.3.3. Liver and Pancreatic Cancers
LncRNA-loaded exosomes from tumors from other organs related to the GI tract have also shown some relationship with tumor angiogenesis. You et al. [101] reported high levels of Linc-00161 in serum-derived exosomes from hepatocellular cancer (HCC) patients and the supernatants of HCC cell lines, which are associated with poor survival. Mechanistically, Linc-00161 promoted angiogenesis in HUVECs by inhibiting miR-590-3p and activating the Rho-associated coiled-coil-containing protein kinase 2 (ROCK2) axis. In an in vitro study, exosomes with lncRNA H19 were released by CD90+ HCC cells and modulated endothelial cells, promoting an angiogenic phenotype and cell-to-cell adhesion [103]. Similarly, lncRNA ubiquitin-conjugating enzyme E2 C pseudogene 3 (UBE2CP3) was overexpressed in HCC EVs. It promoted HUVEC proliferation, migration, and tube formation via the activation of the ERK/HIF-1α/p70S6K/VEGFA signaling cascade, promoting HCC tumorigenicity [102]. In another study, exosomal circ-100388 affected the cell proliferation, angiogenesis, permeability, and vasculogenic mimicry formation ability of HUVECs and HCC tumor metastasis [104]. In cholangiocarcinoma (CCA), the cholangiocarcinoma-associated circular RNA 1 (circ-CCAC1) from CCA-derived EVs was transferred to endothelial monolayer cells, disrupting endothelial barrier integrity and inducing angiogenesis. Interestingly, circ-CCAC1 increased cell leakiness by sequestering the enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) gene, thus elevating the SH3 domain-containing GRB2 like 2, endophilin A1 (SH3GL2) expression to reduce levels of intercellular junction proteins [124]. In pancreatic cancer, the expression levels of the lncRNA urothelial cancer-associated 1 (UCA1) in exosomes derived from the serum of patients were associated with poor survival, promoting angiogenesis and tumor growth by acting as a ceRNA of miR-96-5p, relieving the repressive effects on the expression of its target gene angiomotin like 2 (AMOTL2) [90]. Moreover, the exosomal small nucleolar RNA host gene 11 (SNHG11) promoted cell proliferation, migration, and angiogenesis in pancreatic cancer cell lines but impeded cell apoptosis via sponging miR-324-3p to upregulate VEGFA expression [126].3.4. Renal and Bladder Cancers
Some urinary system tumors have observed a relationship between lncRNA-loaded EVs and angiogenesis. In renal cell carcinoma (RCC), RCC-derived exosomes had an lncRNA Ars operon (ARSR) that promoted macrophage polarization, cytokine release, phagocytosis, angiogenesis, and tumor development by sponging miR34/miR-449 and upregulating the signal transducer and activator of transcription 3 (STAT3) pathway [108]. Similarly, RCC-derived exosomal circular scaffold attachment factor B2 (circ-SAFB2) facilitated the progression, invasion, angiogenesis, and metastasis of RCC by inducing the polarization of M2 macrophages through the miR-620/JAK1/STAT3 axis [109]. Conversely, exosomal circular spire type actin nucleation factor 1 (circ-SPIRE1) suppressed angiogenesis and vessel permeability through regulating ELAV-like RNA-binding protein 1-mRNA, binding and upregulating polypeptide N-Acetylgalactosaminyltransferase 3 (GALNT3) and KH domain RNA-binding protein (QKI) expression [110]. In bladder carcinoma (BCa), exosomal brain cytoplasmic RNA 1 (BCYRN1) promoted the tube formation and migration of human lymphatic endothelial cells (HLECs), upregulating the Wnt family member 5A (WNT5A) gene expression by inducing hnRNPA1-associated H2K4 trimethylation in WNAT5a promoter, which activated Wnt/β-catenin signaling to facilitate the secretion of VEGF-C in BCa [111]. Moreover, lymph node metastasis-associated transcript 2 (LNMAT2)-loaded exosomes from BCa tissues and blood samples stimulated the tube formation and migration of HLECs and enhanced tumor lymphangiogenesis and lymph node metastasis by upregulation of prospero homeobox 1 (PROX1) gene expression by recruitment of hnRNPA2B1 and increasing H3K4 trimethylation [112]. Comparably, BCa cell-secreted EVs mediated intercellular communication with HLECs through the transmission of the small nucleolar RNA host gene 16 (ELNAT1) and promoted lymphangiogenesis by inducing the ubiquitin-conjugating enzyme E2 (UBC9) gene overexpression to catalyze the small ubiquitin-like modifier (SUMO) binding of hnRNPA1 at the lysine 113 residue [113].3.5. Nasopharyngeal and Lung Cancers
LncRNAs in exosomes derived from nasopharyngeal squamous cell carcinoma (NPSCC) and their relationship with tumor angiogenesis have been mildly explored. In serum samples from newly diagnosed NPSCC patients, the long intergenic non-protein-coding RNA, regulator of reprogramming (linc-ROR), was substantially expressed in exosomes that could be taken up by HUVECs, increasing proliferation, migration, and angiogenesis in vitro by mechanistically upregulating the p-AKT/p-VEGFR2 pathway [91]. Similarly, lncRNA colon cancer-associated transcript 2 (CCAT2) was found in EVs derived from NPSCC patients, promoting HUVEC proliferation and angiogenesis promotion [92]. In non-small cell lung carcinoma (NSCLC), NSCLC cells secreted exosomes with melanotransferrin antisense 1 (MFI2-AS1) to induce tube formation by HUVECs, promoting angiogenesis and metastasis by sponging miR-107, which in turn activated the PI3K/AKT pathway [105]. Similarly, high EV Linc-p21 was found in NSCLC blood samples from tumor-draining pulmonary veins before tumor surgical resection. EVs with Linc-p21 were taken up by HUVECs and promoted tube formation and enhanced tumor cell adhesion to endothelial cells by sponging miR-23a, miR-146bv, miR-330, and miR-494 [106]. In contrast, GAS5 was lowly expressed in human lung cancer tissues, lung cancer cells, and cell culture supernatant exosomes. The exosomes of lung cancer cells containing high GAS5 levels inhibited HUVECs proliferation and tube formation, increasing their apoptosis by sponging miR-29-3p and upregulating phosphatase and tensin homolog (PTEN) and inhibiting PI3K/AKT phosphorylation [107].3.6. Glioma and Gliobastoma
A few studies have reported evidence of the role of exosomal lncRNAs in glioma and glioblastoma angiogenesis. An in vitro study with glioma cell lines demonstrated that HUVECs can take up exosomal CCAT2 to promote migration, proliferation, tubular-like structure formation, and arteriole formation [94]. Similarly, the POU class 3 homeobox 3 (POU3F3) was upregulated in glioma tissue. When human brain microvascular endothelial cells (HBMVECs) were treated with exosomes loaded with POU3F3, they exhibited better migration, proliferation, tubular-like structure formation, and arteriole formation. Mechanistically, POU3F3 was shown to upregulate bFGF, bFGFR, VEGFA, and Angio [95]. Moreover, cell line A172 was cultured to demonstrate that EVs loaded with the HOX transcript antisense RNA (HOTAIR) had a pro-angiogenic activity in HBMVECS via VEGFA [96]. In glioblastoma, lncRNA HIF1A antisense RNA 2 (AHIF) was found upregulated in tissue samples, and when cultured with glioblastoma cell lines, exosomal AHIF regulated factors associated with migration and angiogenesis [97].3.7. Other Cancer Types
In ovarian cancer, an in vitro study revealed that lncRNA activated by TGF-β (ATB) promoted viability and angiogenesis of HUVECs by sponging miR-204-3p and thus upregulating TGFβ-R2 [127]. Similarly, elevated serum exosomal metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) promoted angiogenesis and was highly correlated with an advanced and metastatic phenotype of epithelial ovarian cancer [118]. Another study demonstrated taurine-upregulated 1 (TUG1) overexpression in human cervical cancer cell lines. When TUG1 was depleted, the exosome-mediated pro-angiogenic potential of HUVECs was impaired by modulating angiogenesis-related genes like VEGFA, MMP9, TGFβ, IL-8, and bFGF [119]. In breast cancer cell lines, the metadherin (MTDH) gene improved cell viability and angiogenesis in endothelial cells. The molecular cascade was promoted by exosomal circular homeodomain-interacting protein kinase 3 (circ-HIPK3), which sponged miR-124-3p and in turn upregulated MTDH [120]. Liu et al. Campo [121] demonstrated that exosomal overexpression of the FYVE, RhoGF, and PH domain-containing 5 antisense 1 (FGD5-AS1) enhanced the proliferation, migration, angiogenesis, and permeability of HUVECs by regulating the endothelial miR-6838-5p/Vav guanine nucleotide exchange factor 2 (VAV2) axis. A total of 25 peripheral blood samples from 20 multiple myeloma patients and 5 matched healthy controls showed overexpression of the circular ATPase phospholipid-transporting 10A (circ-ATP10A) in the multiple myeloma samples, mechanistically acting as a sponge of several miRNAs to consequently regulate the expression of downstream VEGFB, HIF1A, PDGFA, and FGF [122].References
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer—Review paper. Ann. Agric. Environ. Med. 2019, 26, 1–7.
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79.
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165715.
- Li, S.; Xu, H.X.; Wu, C.T.; Wang, W.Q.; Jin, W.; Gao, H.L.; Li, H.; Zhang, S.R.; Xu, J.Z.; Qi, Z.H.; et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 2019, 22, 15–36.
- Badodekar, N.; Sharma, A.; Patil, V.; Telang, G.; Sharma, R.; Patil, S.; Vyas, N.; Somasundaram, I. Angiogenesis induction in breast cancer: A paracrine paradigm. Cell Biochem. Funct. 2021, 39, 860–873.
- Tan, H.W.; Xu, Y.M.; Qin, S.H.; Chen, G.F.; Lau, A.T.Y. Epigenetic regulation of angiogenesis in lung cancer. J. Cell Physiol. 2021, 236, 3194–3206.
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177.
- Liang, P.; Ballou, B.; Lv, X.; Si, W.; Bruchez, M.P.; Huang, W.; Dong, X. Monotherapy and Combination Therapy Using Anti-Angiogenic Nanoagents to Fight Cancer. Adv. Mater. 2021, 33, e2005155.
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312.
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455.
- Fonseka, P.; Marzan, A.L.; Mathivanan, S. Introduction to the Community of Extracellular Vesicles. Subcell. Biochem. 2021, 97, 3–18.
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39.
- D’Souza-Schorey, C.; Schorey, J.S. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018, 62, 125–133.
- Carnino, J.M.; Lee, H. Extracellular vesicles in respiratory disease. Adv. Clin. Chem. 2022, 108, 105–127.
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273.
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513.
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022, 233, 108025.
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848.
- Chang, C.H.; Pauklin, S. Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death Dis. 2021, 12, 973.
- Wu, H.; Fu, M.; Liu, J.; Chong, W.; Fang, Z.; Du, F.; Liu, Y.; Shang, L.; Li, L. The role and application of small extracellular vesicles in gastric cancer. Mol. Cancer 2021, 20, 71.
- Wang, M.; Zhou, L.; Yu, F.; Zhang, Y.; Li, P.; Wang, K. The functional roles of exosomal long non-coding RNAs in cancer. Cell Mol. Life Sci. 2019, 76, 2059–2076.
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92.
- Entezari, M.; Ghanbarirad, M.; Taheriazam, A.; Sadrkhanloo, M.; Zabolian, A.; Goharrizi, M.; Hushmandi, K.; Aref, A.R.; Ashrafizadeh, M.; Zarrabi, A.; et al. Long non-coding RNAs and exosomal lncRNAs: Potential functions in lung cancer progression, drug resistance and tumor microenvironment remodeling. Biomed. Pharmacother. 2022, 150, 112963.
- Meng, X.; Wang, Z.F.; Lou, Q.Y.; Rankine, A.N.; Zheng, W.X.; Zhang, Z.H.; Zhang, L.; Gu, H. Long non-coding RNAs in head and neck squamous cell carcinoma: Diagnostic biomarkers, targeted therapies, and prognostic roles. Eur. J. Pharmacol. 2021, 902, 174114.
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406.
- Joshi, M.; Rajender, S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod. Biol. Endocrinol. 2020, 18, 103.
- Schmitz, S.U.; Grote, P.; Herrmann, B.G. Mechanisms of long noncoding RNA function in development and disease. Cell Mol. Life Sci. 2016, 73, 2491–2509.
- Lin, W.; Zhou, Q.; Wang, C.Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. LncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194–1206.
- Liu, S.J.; Dang, H.X.; Lim, D.A.; Feng, F.Y.; Maher, C.A. Long noncoding RNAs in cancer metastasis. Nat. Rev. Cancer 2021, 21, 446–460.
- Taghvimi, S.; Abbaszadeh, S.; Banan, F.B.; Fard, E.S.; Jamali, Z.; Najafabadi, M.A.; Savardashtaki, A.; Movahedpour, A. LncRNAs Roles in Chemoresistance of Cancer Cells. Curr. Mol. Med. 2022, 22, 691–702.
- Kichi, Z.A.; Soltani, M.; Rezaei, M.; Shirvani-Farsani, Z.; Rojhannezhad, M. The Emerging Role of EMT-related lncRNAs in Therapy Resistance and their Applications as Biomarkers. Curr. Med. Chem. 2022, 29, 4574–4601.
- Jin, K.T.; Yao, J.Y.; Fang, X.L.; Di, H.; Ma, Y.Y. Roles of lncRNAs in cancer: Focusing on angiogenesis. Life Sci. 2020, 252, 117647.
- Qin, T.; Li, J.; Zhang, K.Q. Structure, Regulation, and Function of Linear and Circular Long Non-Coding RNAs. Front. Genet. 2020, 11, 150.
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172.
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836.
- Peña-Flores, J.A.; Bermúdez, M.; Ramos-Payán, R.; Villegas-Mercado, C.E.; Soto-Barreras, U.; Muela-Campos, D.; Álvarez-Ramírez, A.; Pérez-Aguirre, B.; Larrinua-Pacheco, A.D.; López-Camarillo, C.; et al. Emerging role of lncRNAs in drug resistance mechanisms in head and neck squamous cell carcinoma. Front. Oncol. 2022, 12, 965628.
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. WIREs RNA 2017, 8, e1413.
- Momen-Heravi, F.; Getting, S.J.; Moschos, S.A. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol. Ther. 2018, 192, 170–187.
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532.
- Patan, S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 2004, 117, 3–32.
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092.
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125.
- Wang, F.; Qian, H.; Kong, L.; Wang, W.; Wang, X.; Xu, Z.; Chai, Y.; Xu, J.; Kang, Q. Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2021, 17, 1821–1836.
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347.
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426.
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264.
- Lee, H.W.; Xu, Y.; He, L.; Choi, W.; Gonzalez, D.; Jin, S.W.; Simons, M. Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis. Circulation 2021, 144, 1308–1322.
- Zeng, A.; Wang, S.R.; He, Y.X.; Yan, Y.; Zhang, Y. Progress in understanding of the stalk and tip cells formation involvement in angiogenesis mechanisms. Tissue Cell 2021, 73, 101626.
- Strilić, B.; Kucera, T.; Eglinger, J.; Hughes, M.R.; McNagny, K.M.; Tsukita, S.; Dejana, E.; Ferrara, N.; Lammert, E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 2009, 17, 505–515.
- Mandel, E.R.; Uchida, C.; Nwadozi, E.; Makki, A.; Haas, T.L. Tissue Inhibitor of Metalloproteinase 1 Influences Vascular Adaptations to Chronic Alterations in Blood Flow. J. Cell Physiol. 2017, 232, 831–841.
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106.
- Ribatti, D.; Tamma, R. Hematopoietic growth factors and tumor angiogenesis. Cancer Lett. 2019, 440–441, 47–53.
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19.
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625.
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840.
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312.
- Presta, M.; Andrés, G.; Leali, D.; Dell’Era, P.; Ronca, R. Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur. Cytokine Netw. 2009, 20, 39–50.
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692.
- Parmar, D.; Apte, M. Angiopoietin inhibitors: A review on targeting tumor angiogenesis. Eur. J. Pharmacol. 2021, 899, 174021.
- Rao, L.; Giannico, D.; Leone, P.; Solimando, A.G.; Maiorano, E.; Caporusso, C.; Duda, L.; Tamma, R.; Mallamaci, R.; Susca, N.; et al. HB-EGF-EGFR Signaling in Bone Marrow Endothelial Cells Mediates Angiogenesis Associated with Multiple Myeloma. Cancers 2020, 12, 173.
- Zhang, C.; Zhu, M.; Wang, W.; Chen, D.; Chen, S.; Zheng, H. TNF-α promotes tumor lymph angiogenesis in head and neck squamous cell carcinoma through regulation of ERK3. Transl. Cancer Res. 2019, 8, 2439–2448.
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471.
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920.
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673.
- Ma, Q.; Beal, J.R.; Bhurke, A.; Kannan, A.; Yu, J.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Extracellular vesicles secreted by human uterine stromal cells regulate decidualization, angiogenesis, and trophoblast differentiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2200252119.
- Li, Q.; Xu, Y.; Lv, K.; Wang, Y.; Zhong, Z.; Xiao, C.; Zhu, K.; Ni, C.; Wang, K.; Kong, M.; et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci. Transl. Med. 2021, 13, 584.
- Gregorius, J.; Wang, C.; Stambouli, O.; Hussner, T.; Qi, Y.; Tertel, T.; Börger, V.; Mohamud Yusuf, A.; Hagemann, N.; Yin, D.; et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic. Res. Cardiol. 2021, 116, 40.
- You, B.; Pan, S.; Gu, M.; Zhang, K.; Xia, T.; Zhang, S.; Chen, W.; Xie, H.; Fan, Y.; Yao, H.; et al. Extracellular vesicles rich in HAX1 promote angiogenesis by modulating ITGB6 translation. J. Extracell. Vesicles 2022, 11, e12221.
- Angioni, R.; Liboni, C.; Herkenne, S.; Sánchez-Rodríguez, R.; Borile, G.; Marcuzzi, E.; Calì, B.; Muraca, M.; Viola, A. CD73+ extracellular vesicles inhibit angiogenesis through adenosine A(2B) receptor signalling. J. Extracell. Vesicles 2020, 9, 1757900.
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510.
- Ma, W.; Zhang, W.; Cui, B.; Gao, J.; Liu, Q.; Yao, M.; Ning, H.; Xing, L. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis. 2021, 12, 1056.
- Romano, R.; Picca, A.; Eusebi, L.H.U.; Marzetti, E.; Calvani, R.; Moro, L.; Bucci, C.; Guerra, F. Extracellular Vesicles and Pancreatic Cancer: Insights on the Roles of miRNA, lncRNA, and Protein Cargos in Cancer Progression. Cells 2021, 10, 1361.
- Lai, H.; Li, Y.; Zhang, H.; Hu, J.; Liao, J.; Su, Y.; Li, Q.; Chen, B.; Li, C.; Wang, Z.; et al. exoRBase 2.0: An atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022, 50, D118–D128.
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146.
- Wu, Y.; Wu, X.; Wang, J.; Chen, S.; Chen, H.; Liu, J.; Zeng, T.; Hu, M.; Liang, Y.; Sun, K.; et al. Fibroblast-Derived Extracellular Vesicle-Packaged Long Noncoding RNA Upregulated in Diabetic Skin Enhances Keratinocyte MMP-9 Expression and Delays Diabetic Wound Healing. Lab. Investig. 2023, 103, 100019.
- Yang, Z.; Liu, X.; Zhao, F.; Yao, M.; Lin, Z.; Yang, Z.; Liu, C.; Liu, Y.; Chen, X.; Du, C. Bioactive glass nanoparticles inhibit osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression in the extracellular vesicles derived from bone marrow mesenchymal stem cells. Biomaterials 2022, 283, 121438.
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422.
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204.
- Zhuang, Y.; Liu, K.; He, Q.; Gu, X.; Jiang, C.; Wu, J. Hypoxia signaling in cancer: Implications for therapeutic interventions. MedComm 2023, 4, e203.
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57.
- Jiang, S.; Fu, R.; Shi, J.; Wu, H.; Mai, J.; Hua, X.; Chen, H.; Liu, J.; Lu, M.; Li, N. CircRNA-Mediated Regulation of Angiogenesis: A New Chapter in Cancer Biology. Front. Oncol. 2021, 11, 553706.
- Zhong, Z.; Huang, M.; Lv, M.; He, Y.; Duan, C.; Zhang, L.; Chen, J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017, 403, 305–317.
- Li, Y.; Lin, S.; Xie, X.; Zhu, H.; Fan, T.; Wang, S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am. J. Transl. Res. 2021, 13, 4211–4223.
- Wang, B.D.; Yu, X.J.; Hou, J.C.; Fu, B.; Zheng, H.; Liu, Q.K.; Wang, S.X.; Bi, Z.G.; Cao, Y. Bevacizumab attenuates osteosarcoma angiogenesis by suppressing MIAT encapsulated by serum-derived extracellular vesicles and facilitating miR-613-mediated GPR158 inhibition. Cell Death Dis. 2022, 13, 272.
- Feng, D.; Li, Z.; Yang, L.; Liang, H.; He, H.; Liu, L.; Zhang, W. BMSC-EV-derived lncRNA NORAD Facilitates Migration, Invasion, and Angiogenesis in Osteosarcoma Cells by Regulating CREBBP via Delivery of miR-877-3p. Oxid. Med. Cell Longev. 2022, 2022, 8825784.
- Tao, S.C.; Huang, J.Y.; Wei, Z.Y.; Li, Z.X.; Guo, S.C. EWSAT1 Acts in Concert with Exosomes in Osteosarcoma Progression and Tumor-Induced Angiogenesis: The “Double Stacking Effect”. Adv. Biosyst. 2020, 4, e2000152.
- Cheng, C.; Zhang, Z.; Cheng, F.; Shao, Z. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. Onco Targets Ther. 2020, 13, 3291–3301.
- Guo, Z.; Wang, X.; Yang, Y.; Chen, W.; Zhang, K.; Teng, B.; Huang, C.; Zhao, Q.; Qiu, Z. Hypoxic Tumor-Derived Exosomal Long Noncoding RNA UCA1 Promotes Angiogenesis via miR-96-5p/AMOTL2 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2020, 22, 179–195.
- Zhang, S.; Cai, J.; Ji, Y.; Zhou, S.; Miao, M.; Zhu, R.; Li, K.; Xue, Z.; Hu, S. Tumor-derived exosomal lincRNA ROR promotes angiogenesis in nasopharyngeal carcinoma. Mol. Cell Probes 2022, 66, 101868.
- Zhou, S.K.; Gao, F.; Zhong, Z.S.; Yao, H. Long non-coding RNA colon cancer associated transcript-2 from nasopharyngeal carcinoma-derived exosomes promotes angiogenesis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 944–951.
- Zhang, C.; Luo, Y.; Cao, J.; Wang, X.; Miao, Z.; Shao, G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020, 9, 8600–8611.
- Lang, H.L.; Hu, G.W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017, 38, 785–798.
- Lang, H.L.; Hu, G.W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.M.; Wu, L.; Xu, G.H. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972.
- Ma, X.; Li, Z.; Li, T.; Zhu, L.; Li, Z.; Tian, N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 2017, 9, 5012–5021.
- Dai, X.; Liao, K.; Zhuang, Z.; Chen, B.; Zhou, Z.; Zhou, S.; Lin, G.; Zhang, F.; Lin, Y.; Miao, Y.; et al. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int. J. Oncol. 2019, 54, 261–270.
- Zeng, W.; Liu, Y.; Li, W.T.; Li, Y.; Zhu, J.F. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol. Oncol. 2020, 14, 2960–2984.
- Wang, F.W.; Cao, C.H.; Han, K.; Zhao, Y.X.; Cai, M.Y.; Xiang, Z.C.; Zhang, J.X.; Chen, J.W.; Zhong, L.P.; Huang, Y.; et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J. Clin. Investig. 2019, 129, 727–743.
- Chen, C.; Liu, Y.; Liu, L.; Si, C.; Xu, Y.; Wu, X.; Wang, C.; Sun, Z.; Kang, Q. Exosomal circTUBGCP4 promotes vascular endothelial cell tipping and colorectal cancer metastasis by activating Akt signaling pathway. J. Exp. Clin. Cancer Res. 2023, 42, 46.
- You, L.N.; Tai, Q.W.; Xu, L.; Hao, Y.; Guo, W.J.; Zhang, Q.; Tong, Q.; Zhang, H.; Huang, W.K. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021, 28, 719–736.
- Lin, J.; Cao, S.; Wang, Y.; Hu, Y.; Liu, H.; Li, J.; Chen, J.; Li, P.; Liu, J.; Wang, Q.; et al. Long non-coding RNA UBE2CP3 enhances HCC cell secretion of VEGFA and promotes angiogenesis by activating ERK1/2/HIF-1α/VEGFA signalling in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 113.
- Conigliaro, A.; Costa, V.; Lo Dico, A.; Saieva, L.; Buccheri, S.; Dieli, F.; Manno, M.; Raccosta, S.; Mancone, C.; Tripodi, M.; et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 2015, 14, 155.
- Huang, X.Y.; Huang, Z.L.; Huang, J.; Xu, B.; Huang, X.Y.; Xu, Y.H.; Zhou, J.; Tang, Z.Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20.
- Xu, J.; Wang, H.; Shi, B.; Li, N.; Xu, G.; Yan, X.; Xu, L. Exosomal MFI2-AS1 sponge miR-107 promotes non-small cell lung cancer progression through NFAT5. Cancer Cell Int. 2023, 23, 51.
- Castellano, J.J.; Marrades, R.M.; Molins, L.; Viñolas, N.; Moises, J.; Canals, J.; Han, B.; Li, Y.; Martinez, D.; Monzó, M.; et al. Extracellular Vesicle lincRNA-p21 Expression in Tumor-Draining Pulmonary Vein Defines Prognosis in NSCLC and Modulates Endothelial Cell Behavior. Cancers 2020, 12, 734.
- Cheng, Y.; Dai, X.; Yang, T.; Zhang, N.; Liu, Z.; Jiang, Y. Low Long Noncoding RNA Growth Arrest-Specific Transcript 5 Expression in the Exosomes of Lung Cancer Cells Promotes Tumor Angiogenesis. J. Oncol. 2019, 2019, 2476175.
- Zhang, W.; Zheng, X.; Yu, Y.; Zheng, L.; Lan, J.; Wu, Y.; Liu, H.; Zhao, A.; Huang, H.; Chen, W. Renal cell carcinoma-derived exosomes deliver lncARSR to induce macrophage polarization and promote tumor progression via STAT3 pathway. Int. J. Biol. Sci. 2022, 18, 3209–3222.
- Huang, X.; Wang, J.; Guan, J.; Zheng, Z.; Hao, J.; Sheng, Z.; Wang, M.; Xu, T.; Guo, G.; Yao, L. Exosomal Circsafb2 Reshaping Tumor Environment to Promote Renal Cell Carcinoma Progression by Mediating M2 Macrophage Polarization. Front. Oncol. 2022, 12, 808888.
- Shu, G.; Lu, X.; Pan, Y.; Cen, J.; Huang, K.; Zhou, M.; Lu, J.; Dong, J.; Han, H.; Chen, W.; et al. Exosomal circSPIRE1 mediates glycosylation of E-cadherin to suppress metastasis of renal cell carcinoma. Oncogene 2023, 42, 1802–1820.
- Zheng, H.; Chen, C.; Luo, Y.; Yu, M.; He, W.; An, M.; Gao, B.; Kong, Y.; Ya, Y.; Lin, Y.; et al. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin. Transl. Med. 2021, 11, e497.
- Chen, C.; Luo, Y.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.; Zhong, G.; Li, Y.; Li, J.; Huang, J.; et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020, 130, 404–421.
- Chen, C.; Zheng, H.; Luo, Y.; Kong, Y.; An, M.; Li, Y.; He, W.; Gao, B.; Zhao, Y.; Huang, H.; et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J. Clin. Investig. 2021, 131, 8.
- Liu, C.; Yang, J.; Zhu, F.; Zhao, Z.; Gao, L. Exosomal circ_0001190 Regulates the Progression of Gastric Cancer via miR-586/SOSTDC1 Axis. Biochem. Genet. 2022, 60, 1895–1913.
- Li, S.; Li, J.; Zhang, H.; Zhang, Y.; Wang, X.; Yang, H.; Zhou, Z.; Hao, X.; Ying, G.; Ba, Y. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem. Biophys. Res. Commun. 2021, 560, 37–44.
- Xie, M.; Yu, T.; Jing, X.; Ma, L.; Fan, Y.; Yang, F.; Ma, P.; Jiang, H.; Wu, X.; Shu, Y.; et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer 2020, 19, 112.
- Zhang, Z.; Sun, C.; Zheng, Y.; Gong, Y. circFCHO2 promotes gastric cancer progression by activating the JAK1/STAT3 pathway via sponging miR-194-5p. Cell Cycle 2022, 21, 2145–2164.
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Lin, Y.Y.; Hua, K.Q. Exosomal Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973.
- Lei, L.; Mou, Q. Exosomal taurine up-regulated 1 promotes angiogenesis and endothelial cell proliferation in cervical cancer. Cancer Biol. Ther. 2020, 21, 717–725.
- Shi, P.; Liu, Y.; Yang, H.; Hu, B. Breast cancer derived exosomes promoted angiogenesis of endothelial cells in microenvironment via circHIPK3/miR-124-3p/MTDH axis. Cell Signal 2022, 95, 110338.
- Liu, B.; Chen, J.; Shang, F.; Lian, M.; Shen, X.; Fang, J. Tumor-Derived Exosome FGD5-AS1 Promotes Angiogenesis, Vascular Permeability, and Metastasis in Thyroid Cancer by Targeting the miR-6838-5p/VAV2 Axis. J. Oncol. 2022, 2022, 4702855.
- Yu, M.; Yu, J.; Zhang, Y.; Sun, X.; Sun, R.; Xia, M.; Li, S.; Cui, X. A novel circRNA-miRNA-mRNA network revealed exosomal circ-ATP10A as a biomarker for multiple myeloma angiogenesis. Bioengineered 2022, 13, 667–683.
- Lamichhane, T.N.; Leung, C.A.; Douti, L.Y.; Jay, S.M. Ethanol Induces Enhanced Vascularization Bioactivity of Endothelial Cell-Derived Extracellular Vesicles via Regulation of MicroRNAs and Long Non-Coding RNAs. Sci. Rep. 2017, 7, 13794.
- Xu, Y.; Leng, K.; Yao, Y.; Kang, P.; Liao, G.; Han, Y.; Shi, G.; Ji, D.; Huang, P.; Zheng, W.; et al. A Circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, Contributes to Cholangiocarcinoma Progression, Induces Angiogenesis, and Disrupts Vascular Endothelial Barriers. Hepatology 2021, 73, 1419–1435.
- Zhi, J.; Jia, X.J.; Yan, J.; Wang, H.C.; Feng, B.; Xing, H.Y.; Jia, Y.T. BRAF(V600E) mutant colorectal cancer cells mediate local immunosuppressive microenvironment through exosomal long noncoding RNAs. World J. Gastrointest. Oncol. 2021, 13, 2129–2148.
- Fang, X.; Cai, Y.; Xu, Y.; Zhang, H. Exosome-mediated lncRNA SNHG11 regulates angiogenesis in pancreatic carcinoma through miR-324-3p/VEGFA axis. Cell Biol. Int. 2022, 46, 106–117.
- Yuan, D.; Guo, T.; Zhu, D.; Ge, H.; Zhao, Y.; Huang, A.; Wang, X.; Cao, X.; He, C.; Qian, H.; et al. Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promotes Angiogenesis via Regulating miR-204-3p/TGFβR2 Axis. Cancer Manag. Res. 2022, 14, 327–337.
More
