Infective endocarditis (IE) is still a life-threatening disease with frequent lethal outcomes despite the profound changes in its clinical, microbiological, imaging, and therapeutic profiles. Nowadays, the scenario for IE has changed since rheumatic fever has declined, but on the other hand, multiple aspects, such as elderly populations, cardiovascular device implantation procedures, and better use of multiple imaging modalities and multidisciplinary care, have increased, leading to escalations in diagnosis. Mainly, guidelines put emphasis on the importance of an endocarditis team in the handling of systemic complications and how they affect the timing of surgery and perioperative management. Neurological complications, acute renal failure, splenic or musculoskeletal manifestations, or infections determined by multiresistant microorganisms or fungi can affect long-term prognosis and survival. Not to be outdone, anatomical and surgical factors, such as the presence of native or prosthetic valve endocarditis, a repair strategy when feasible, anatomical extension and disruption in the case of an annular abscess (mitral valve annulus, aortic mitral curtain, aortic root, and annulus), and the choice of prosthesis and conduits, can be equally crucial.
- infective endocarditis
- native valve endocarditis
- prosthetic valve endocarditis
1. Introduction
2. Native Valve Endocarditis: Repair versus Replacement
Native valve infective endocarditis is not frequently encountered, with an estimated occurrence of about 2 to 10 instances per 100,000 person-years [7][4]. Damage to the valvular endothelium or endocardium exposes subendothelial collagen and matrix molecules, prompting platelet and fibrin accumulation and forming a microthrombotic lesion. Subsequently, bacteria present in the bloodstream colonize this lesion, initiating replication and leading to the development of an infected vegetation, a defining characteristic of infective endocarditis. Certain cardiac conditions can predispose individuals to the onset of infective endocarditis, such as congenital anomalies (like ventricular septal defects and bicuspid aortic valves) or acquired valvular issues (including degenerative valvular diseases, aortic stenosis, and rheumatic heart disease). In more developed nations, the principal predisposing cardiac conditions encompass degenerative valvular disorders, congenital valvular irregularities, and the presence of intracardiac devices [8][5]. On the other hand, factors not directly related to the heart, such as poor dental health, intravenous drug usage, hemodialysis, chronic liver disease, diabetes, compromised immune function, neoplastic diseases, and the presence of indwelling intravascular devices, can also contribute to an individual’s susceptibility to this condition. The general principle of IE surgery is to obtain a radical debridement of vegetation and infected tissue and to avoid and limit, when possible, the use of foreign material. When it comes to infective endocarditis involving native heart valves, the mitral valve (MV) tends to be affected most frequently, accounting for approximately 40–50% of cases [9][6]. Throughout history, mitral valve repair (MVr) has demonstrated better survival rates and overall outcomes, especially in comparison to valve replacement (MVR), effectively restoring life expectancy across all age brackets. Furthermore, a strong association has been observed between surgical volume and patient outcomes, both at hospital and surgeon levels. This suggests that the frequency of interventions performed by a surgeon is an independent predictor of successful repair, surgical outcomes, and enhanced survival [10][7]. However, within the context of endocarditis, the advantages and predictors of mitral valve repair are less well established. Within this particularly challenging and intricate patient population, real-world statistics from multicenter reports indicate that rates of successful repair range only from 19% to 32% [11][8]. In general, techniques may vary between leaflet segmental resection for valve destruction, primary closure, or patch repair (Figure 1) for leaflet perforation and neochordae implantation to replace ruptured chordae and support reconstructed leaflets.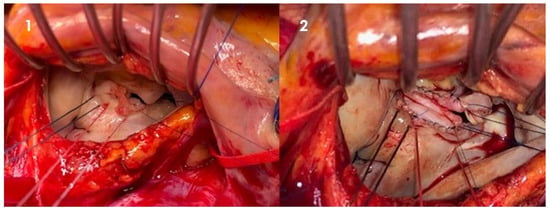
3. Prosthetic Valve and Implantable Device Endocarditis
4. Surgery in Complex Infective Endocarditis: Current Trends
Endocarditis is a potentially destructive disease and the treatment can widely range in complexity from medical antimicrobial treatment to complex cardiac reconstruction. Endocarditic lesions may manifest as small vegetations originating from the cusps or leaflets, or fenestrations, which can often be easily treated with excision and valve replacement or valve repair, as already mentioned. If the disease progresses locally, it may lead to valvular annulus involvement and the development of an annular abscess. In turn, a deeper myocardial involvement may lead to damage to the fibrous skeleton of the heart and to the conduction system, as well as perforation into other cardiac chambers. The scope and nature of surgical intervention are directly contingent upon the extent of tissue damage, consistently adhering to the principle of thorough debridement, and may necessitate annular reconstruction. The choice of material for the new annulus is generally a matter of surgeon preference, with options including bovine pericardium, both fixed and fresh autologous pericardium, as well as Dacron, all being documented choices. Pericardial tissue holds the advantage of increased pliability and the capacity to conform to the underlying myocardium, thereby reducing the likelihood of flow beneath the patch that could potentially lead to the development of a pseudoaneurysm or shunt. Additionally, the utilization of autologous or heterologous pericardium has not demonstrated an elevated risk of reinfection. Following the placement of the patch, the decision between valve repair and replacement is determined by the extent of valve leaflet or cusp destruction. Approximately 15% of cases of mitral valve infective endocarditis (MVE) may involve a mitral annular abscess [54][31]. These abscesses are typically located in the posterior mitral annulus, encompassing the posterior leaflet, the annulus itself, and the underlying myocardium. When an annular abscess is present or substantial destruction of the posterior annulus is evident, this implies the necessity of debridement and subsequent reconstruction of the atrioventricular groove using a patch, prior to proceeding with mitral valve surgery. The patch aims to reconstruct atrioventricular continuity and should always be oversized in order to comfortably cover the defect circumferentially. Attention must be paid to the underlying left circumflex coronary artery. Oversizing the patch guarantees good sealing, thanks to the intracavitary ventricular pressure against the myocardium, and contributes to hemostasis. Aortic annular erosion and abscess can be detected in both native and prosthetic aortic valve endocarditis (Figure 32).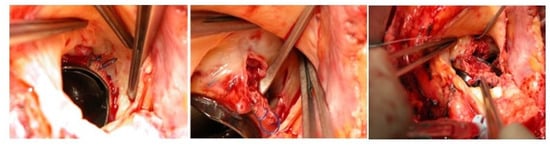
5. Conclusions
References
- Cahill, T.J.; Prendergast, B.D. Infective Endocarditis. Lancet 2016, 387, 882–893.
- de Sa, D.D.C.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; Bachuwar, A.; Pazdernik, M.; Steckelberg, J.M.; Wilson, W.R.; et al. Epidemiological trends of infective endocarditis: A population-based study in Olmsted County, Minnesota. Mayo Clin. Proc. 2010, 85, 422–426.
- Prendergast, B.D.; Tornos, P. Surgery for infective endocarditis: Who and when? Circulation 2010, 121, 1141–1152.
- Chambers, H.F.; Bayer, A.S. Native-Valve infective endocarditis. N. Engl. J. Med. 2020, 383, 567–576.
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473.
- Hill, E.; Herijgers, P.; Herregods, M.-C.; Peetermans, W. Evolving trends in infective endocarditis. Clin. Microbiol. Infect. 2006, 12, 5–12.
- Brescia, A.A.; Watt, T.M.; Rosenbloom, L.M.; Williams, A.M.; Bolling, S.F.; Romano, M.A.; Ailawadi, G.; Wagner, C.M.; Bergquist, C.S.; Murray, S.L.; et al. Patient and Surgeon Predictors of Mitral and Tricuspid Valve Repair for Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 67–77.
- Harky, A.; Hof, A.; Garner, M.; Froghi, S.; Bashir, M. Mitral valve repair or replacement in native valve endocarditis? Systematic review and meta-analysis. J. Card. Surg. 2018, 33, 364–371.
- Toyoda, N.; Itagaki, S.; Egorova, N.N.; Tannous, H.; Anyanwu, A.C.; El-Eshmawi, A.; Adams, D.H.; Chikwe, J. Real-world outcomes of surgery for native mitral valve endocarditis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1906–1912.
- Feringa, H.H.; Shaw, L.J.; Poldermans, D.; Hoeks, S.; van der Wall, E.E.; Dion, R.A.; Bax, J.J. Mitral valve repair and replacement in endocarditis: A systematic review of literature. Ann. Thorac. Surg. 2007, 83, 564–570.
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3233.
- Mayer, K.; Aicher, D.; Feldner, S.; Kunihara, T.; Schafers, H.J. Repair versus replacement of the aortic valve in active infective endocarditis. Eur. J. Cardiothorac. Surg. 2012, 42, 122–127.
- Aicher, D.; Kunihara, T.; Abou Issa, O.; Brittner, B.; Gräber, S.; Schäfers, H.J. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011, 123, 178–185.
- Boodhwani, M.; de Kerchove, L.; Glineur, D.; Poncelet, A.; Rubay, J.; Astarci, P.; Verhelst, R.; Noirhomme, P.; El Khoury, G. Repair-oriented classification of aortic insufficiency: Impact on surgical techniques and clinical outcomes. J. Thorac. Cardiovasc. Surg. 2009, 137, 286–294.
- Shmueli, H.; Thomas, F.; Flint, N.; Setia, G.; Janjic, A.; Siegel, R.J. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J. Am. Heart. Assoc. 2020, 9, e017293.
- Seratnahaei, A.; Leung, S.W.; Charnigo, R.J.; Cummings, M.S.; Sorrell, V.L.; Smith, M.D. The changing “face” of endocarditis in Kentucky: An increase in tricuspid cases. Am. J. Med. 2014, 127, 786.e1–786.e6.
- Yanagawa, B.; Elbatarny, M.; Verma, S.; Hill, S.; Mazine, A.; Puskas, J.D.; Friedrich, J.O. Surgical Management of Tricuspid Valve Infective Endocarditis: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 708–714.
- Divekar, A.A.; Scholz, T.; Fernandez, J.D. Novel percutaneous transcatheter intervention for refractory active endocarditis as a bridge to surgery-AngioVac aspiration system. Catheter. Cardiovasc. Interv. 2013, 81, 1008–1012.
- George, B.; Voelkel, A.; Kotter, J.; Leventhal, A.; Gurley, J. A novel approach to percutaneous removal of large tricuspid valve vegetations using suction filtration and veno-venous bypass: A single center experience. Catheter. Cardiovasc. Interv. 2017, 90, 1009–1015.
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Bärwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective endocarditis in Europe: Lessons from the Euro heart survey. Heart 2005, 91, 571–575.
- Hoen, B.; Alla, F.; Selton-Suty, C.; Béguinot, I.; Bouvet, A.; Briançon, S.; Casalta, J.-P.; Danchin, N.; Delahaye, F.; Etienne, J.; et al. Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA 2002, 288, 75–81.
- Della Corte, A.; Di Mauro, M.; Dato, G.A.; Barili, F.; Cugola, D.; Gelsomino, S.; Santè, P.; Carozza, A.; Della Ratta, E.; Galletti, L.; et al. Surgery for prosthetic valve endocarditis: A retrospective study of a national registry. Eur. J. Cardiothorac. Surg. 2017, 52, 105–111.
- Østergaard, L.; Valeur, N.; Ihlemann, N.; Smerup, M.H.; Bundgaard, H.; Gislason, G.; Torp-Pedersen, C.; Bruun, N.E.; Køber, L.; Fosbøl, E.L. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur. Heart J. 2018, 39, 2668–2675.
- Grover, F.L.; Cohen, D.J.; Oprian, C.; Henderson, W.G.; Sethi, G.; Hammermeister, K. Determinants of the occurrence of and survival from prosthetic valve endocarditis. Experience of the veterans affairs cooperative study on valvular heart disease. J. Thorac. Cardiovasc. Surg. 1994, 108, 207–214.
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Paré, C.; Almirante, B.; Muñoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361.
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: Results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation 2013, 127, 1647–1655.
- Amat-Santos, I.J.; Ribeiro, H.B.; Urena, M.; Allende, R.; Houde, C.; Bédard, E.; Perron, J.; DeLarochellière, R.; Paradis, J.-M.; Dumont, E.; et al. Prosthetic valve endocarditis after transcatheter valve replacement. JACC Cardiovasc. Interv. 2015, 8, 334–346.
- Kolte, D.; Goldsweig, A.; Kennedy, K.F.; Abbott, J.D.; Gordon, P.C.; Sellke, F.W.; Ehsan, A.; Sodha, N.; Sharaf, B.L.; Aronow, H.D. Comparison of incidence, predictors, and outcomes of early infective endocarditis after transcatheter aortic valve implantation versus surgical aortic valve replacement in the United States. Am. J. Cardiol. 2018, 122, 2112–2119.
- Bjursten, H.; Rasmussen, M.; Nozohoor, S.; Götberg, M.; Olaison, L.; Rück, A.; Ragnarsson, S. Infective endocarditis after transcatheter aortic valve implantation: A nationwide study. Eur. Heart J. 2019, 40, 3263–3269.
- Boeder, N.F.; Dörr, O.; Rixe, J.; Weipert, K.; Bauer, T.; Bayer, M.; Hamm, C.W.; Nef, H.M. Endocarditis after interventional repair of the mitral valve: Review of a dilemma. Cardiovasc. Revasc. Med. 2017, 18, 141–144.
- Nataf, P.; Jault, F.; Dorent, R.; Vaissier, E.; Bors, V.; Pavie, A.; Cabrol, C.; Gandjbakhch, I. Extra-annular procedures in the surgical management of prosthetic valve endocarditis. Eur. Heart J. 1995, 16, 99–102.
- Perrotta, S.; Lentini, S. Surgical management of severe damage of the aortic annulus. Hell. J. Cardiol. 2016, 57, 382–388.
- David, T.E.; Kuo, J.; Armstrong, S. Aortic and mitral valve replacement with reconstruction of the intervalvular fibrous body. J. Thorac. Cardiovasc. Surg. 1997, 114, 766–771.
- Davierwala, P.M.; Binner, C.; Subramanian, S.; Luehr, M.; Pfannmueller, B.; Etz, C.; Dohmen, P.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Double valve replacement and reconstruction of the intervalvular fibrous body in patients with active infective endocarditis. Eur. J. Cardiothorac. Surg. 2014, 45, 146–152.
- Navia, J.L.; Al-Ruzzeh, S.; Gordon, S.; Fraser, T.; Agüero, O.; Rodríguez, L. The incorporated aortomitral homograft: A new surgical option for double valve endocarditis. J. Thorac. Cardiovasc. Surg. 2010, 139, 1077–1081.
- Greco, R.; Muretti, M.; Djordjevic, J.; Jin, X.Y.; Hill, E.; Renna, M.; Petrou, M. Surgical Complexity and Outcome of Patients Undergoing Re-do Aortic Valve Surgery. Open Heart 2020, 7, e001209.
