Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by José Alberto Peña Flores Portillo and Version 2 by Camila Xu.
Extracellular vesicles (EVs) are defined as subcellular structures limited by a bilayer lipid membrane that function as important intercellular communication by transporting active biomolecules, such as proteins, amino acids, metabolites, and nucleic acids, including long non-coding RNAs (lncRNAs). These cargos can effectively be delivered to target cells and induce a highly variable response. LncRNAs are functional RNAs composed of at least 200 nucleotides that do not code for proteins. The angiogenesis process is fundamental for cancer to advance locally and facilitate metastasis, and therefore, it has been extensively studied in most cancer types.
- exosomes
- lncRNAs
- long non-coding RNAs
- circRNAs
- cancer
- angiogenesis
1. Introduction
1.1. Cancer Generalities
Cancer is defined as a group of diseases that are multifactorial in nature and represent a challenge in their diagnosis and treatment due to their etiological diversity [1]. More than 200 types of human cancer have been identified based on the cell or tissue from where they originate, the somatic mutations acquired at any time of the progression of the disease, and the microenvironment influences in which they develop [2]. One of the hallmark features of cancer is its rapid and uncontrolled progression due to mutations that alter the cell cycle and overpass checkpoint regulation between the cell cycle phases, promoting the accumulation of mutations passed down to the progeny [3]. For this rapid progression to occur, the growing tumor has a high demand for nutrients and other components; thus, these cells generate molecular signaling to promote the formation of new blood vessels from preexisting ones, a process denominated angiogenesis [4]. The angiogenesis process is fundamental for cancer to advance locally and facilitate metastasis, and therefore, it has been extensively studied in most cancer types [5][6][7][5,6,7]. Multiple efforts have been made to develop antiangiogenic therapies to halt tumor growth and prevent metastasis [8][9][8,9]. Recently, the role of extracellular vesicles (EVs) between vessels in tumor communication has triggered the interest of many researchers.
1.2. Extracellular Vesicles
Extracellular vesicles are subcellular structures that are heterogeneous in nature and surrounded by a lipid bilayer membrane that exerts multiple functions in intercellular communication [10]. Based on how they are delivered from the original cell to the extracellular medium, EVs can be released by inward budding of the endosomal membrane or outward budding of the cellular membrane [11]. The recipient cell can then internalize EVs through endocytosis or membrane fusion to unload their contents into the cell cytoplasm (Figure 1) [12]. Since their discovery in the early 1980s, many biomolecules have been identified as cargo in EVs, including proteins, amino acids, signaling lipids, and different genetic molecules like DNA, RNA, and non-coding RNAs, promoting both physiological and pathological processes [13]. Based on their biogenesis, EVs are generally classified into microvesicles and exosomes [10], although some authors suggest a further division into apoptotic bodies and proteasomes [14]. Microvesicles are generated by outward budding of the plasma membrane and range from 50 nm to 1000 nm. In contrast, exosomes are membrane vesicles smaller in size (30–100 nm) and are formed by inward budding of the endosomal membrane to be later secreted by fusion with the cell membrane [14][15][14,15]. The role of EVs as cell-to-cell mediators in respiratory disease [16], neurodegenerative disease [17], kidney disease [18], cardiovascular disease [19], and cancer progression and metastasis [20][21][22][20,21,22] has been documented to ameliorate the understanding of the behavior or these subcellular structures.
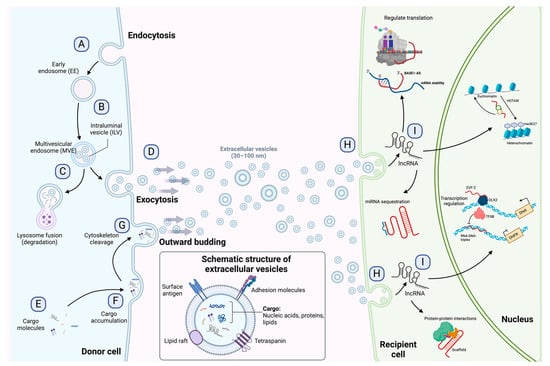
Figure 1. Exosome biogenesis, release to the extracellular environment, and uptake by the recipient cell. (A) Exosome biogenesis begins with early endosome formation during endocytosis. (B) Early endosomes are then matured into late endosomes, generating multiple intraluminal vesicles (ILVs) by the inward budding of endosomal membranes. (C) The accumulation of ILVs leads to the formation of multivesicular endosomes (MVEs), and proteins and nucleic acids produced by the donor cell can be sorted into exosomes during MVE formation. (D) Exosomes are released into the extracellular environment by fusing MVEs with the cellular membrane. (E–G) Microvesicles arise from the outward budding and shedding of the plasma membrane. (H) Extracellular vesicles are taken up by the recipient cell by direct fusion, receptor-mediated fusion, or endocytosis. (I) Exosomal lncRNAs can be subsequently delivered to the recipient cell to exert regulatory effects as sponges for miRNAs, protein scaffolds, transcription and translation regulators, and chromatin activators. The detailed functions of lncRNAs are depicted in Figure 2. Figure 1 is modified from Wang et al. [23].
1.3. Long Non-Coding and circRNAs
Long non-coding RNAs (lncRNAs) are a diverse group of RNAs that are not translated into proteins, and they are at least 200 nucleotides in length [24]. Recent advances in genomic sciences through RNA sequencing have offered the identification of lncRNAs performing functions to control chromatin complexes, recruit transcription factors, regulate alternative splicing, affect mRNA translation, sponge micro-RNAs by binding, degrade other RNAs, and serve as scaffolds for protein interactions (Figure 2) [25][26][27][25,26,27]. Evidence suggests an active role of lncRNAs in most physiological processes, and their involvement in disease has been the focus of active research in recent years [28][29][28,29]. The involvement of lncRNAs as oncogenes or tumor suppressors in many cancer types has also been documented. However, as new lncRNAs are discovered, the general landscape becomes complicated as the roles they can perform become more complex [30]. For instance, lncRNAs can influence the progression of cancer by promoting metastasis [31], drug resistance [32], epithelial-to-mesenchymal transition (EMT) [33], and angiogenesis [34].
It has recently been shown that some lncRNAs can take a circular shape and join covalently at the ends, and these are called circRNAs [35]. This type of lncRNA can perform similar functions to linear lncRNAs, as sponges to recruit specific miRNAs or as effectors to regulate the expression of certain genes [36]. circRNAs have recently been widely studied, arousing interest due to their stability, since, unlike linear non-coding RNAs, they are difficult to degrade [37].
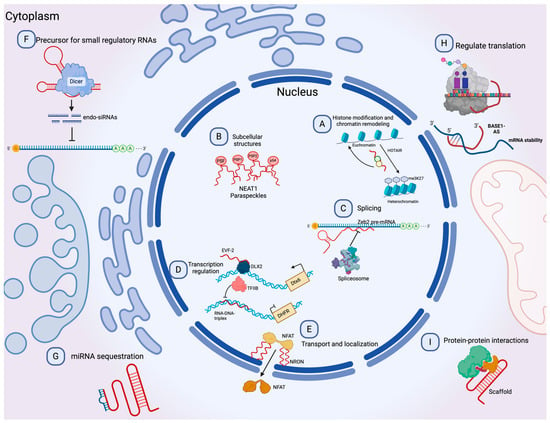
Figure 2. Molecular functions of lncRNAs. (A) lncRNAs can guide chromatin complexes, controlling between transcriptionally active euchromatin and silent heterochromatin. (B) The recruitment of polymerase II and transcription factors can be inhibited or facilitated by lncRNAs. (C) lncRNAs contribute to transcriptome complexity by regulating alternative splicing of pre-mRNAs. (D) lncRNAs affect the stability and translation of mRNA by base-pairing with mRNA molecules. (E) They influence the expression of miRNAs by binding to them and preventing their function. (F) lncRNAs can act as siRNAs and target other RNAs, which subsequently could result in target degradation. (G) lncRNAs can join multiple protein factors as flexible scaffolds to interact with or cooperate in protein–protein interactions. (H,I) The scaffold function is also important for protein activity and localization as well as subcellular structures. Modified from Peña-Flores et al. [38].
Recently, the presence of both coding and non-coding RNA in EVs has motivated research to elucidate RNA’s role in various biological mechanisms in cancer and other diseases [39][40][39,40].
2. Mechanism of Angiogenesis
The angiogenesis process embodies forming new blood vessels from existing vessels in response to physiological and pathological mechanisms [41]. During embryogenesis, the vascular network develops through a combination of vasculogenesis, referred to as the de novo formation of the heart and new blood vessels from stem endothelial cells, namely, angioblasts, and angiogenesis, which expands the initial primitive vascular plexus [42]. Although most blood vessels remain quiescent under physiological conditions, tissue repair and regeneration through wound healing, ovulation, and endometrial thickening throughout the menstrual cycle are based on angiogenesis for proper functioning [43][44][45][43,44,45]. While vascular growth varies depending on where angiogenesis is initiated and the tissue to which they will provide a new blood supply, several mechanisms are common in forming these vessels [46]. In a hypoxic state, the recruitment of cells that promote inflammation; angiogenic growth factor production; degradation of the basement membrane; and endothelial cells (ECs) sprouting, migrating, proliferating, differentiating, and modulating vascular support cells are some of the shared characteristics in angiogenesis [47]. The angiogenic process (Figure 3) comprises several stages involving the sprouting, migration, and proliferation of ECs guided by the vascular endothelial growth factor (VEGF) [48]. Following VEGF stimulation, pericytes from the vessel wall detach, and the basal membrane is weakened by proteolytic degradation. At the same time, ECs adopt an invasive and motile phenotype called tip cells that send out filamentous pseudopodia to guide vascular budding [49]. The cells behind the tip cells are denominated stalk cells, which proliferate to maintain the integrity of the structure and function of the nascent vessels, mainly expanding the vascular lumen [50]. ECs modify their shape by negatively charging glycoproteins on the apical surface to repel each other and open the lumen while redistributing cell-to-cell adhesion to the periphery [51]. For maturation to occur, pericytes must be recruited by the platelet-derived growth factor subunit B (PDGF-B) and angiopoietin 1 (Ang1) signaling along with the strengthening and consolidation of the adhesion between ECs with junctional molecules such as VE-cadherin, while a basement membrane is deposited by tissue inhibitors of metalloproteinases (TIMPs) [52][53][52,53].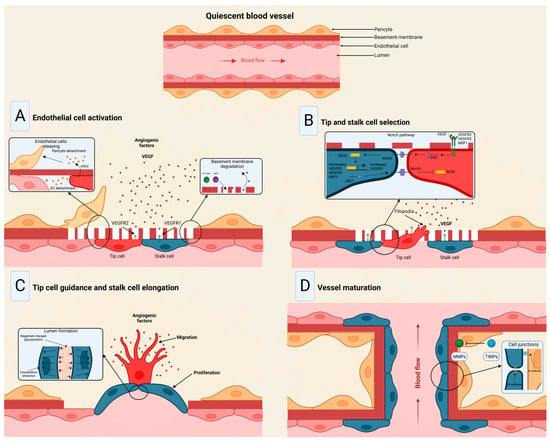
Figure 3. Stages of the angiogenic process. (A) Angiogenic signals, such as VEGF, promote pericyte detachment from the basement membrane and weaken the extracellular matrix. (B) Endothelial cells display characteristic phenotypes after VEGF stimulation: migratory tip cells or proliferating stalk cells. (C) Attractive and repulsive forces control endothelial cells, forming a vessel lumen to initiate blood flow. (D) PDGF-B and Ang1 signaling lead to pericyte recruitment, while junctional molecules consolidate EC–EC adhesion. Modified from Viallard et al. [47].
3. Exosomal Long Non-Coding and Circular RNAs in Cancer Angiogenesis
LncRNAs in EV cargo have been demonstrated lately, mainly in cancer [72][73][74][72,73,74]. A recent study launched an online repository of EV long RNAs (exLRs) in diverse human body fluids, comprising 19,643 mRNAs, 15,645 lncRNAs, and 79,084 circRNAs obtained from human blood, cerebrospinal fluid, bile, and urine samples. The database provides novel exLR signatures to help discover new biomarkers that could aid in diagnosing and treating many diseases [75]. Based on available recent research, Casado-Díaz et al. [76] concluded that lncRNAs and other RNAs included in MSC-derived EVs can be applied in chronic skin ulcers to improve accelerated healing and decrease scar formation due to immunosuppressive and immunomodulatory properties. Conversely, in a diabetic wound-healing animal model, upregulated lncRNAs packed in EVs from fibroblasts enhanced keratinocyte MMP-9 expression to induce collagen degradation, delaying wound healing [77]. Recently, the long non-coding repressor of NFAT (NRON) was detected in BMSC-derived EVs, inhibiting osteoclast differentiation and osteoporotic bone loss in vitro and in vivo [78]. In tumors, the high rate of cell proliferation forces the formation of new blood vessels [79]. However, in most cases, these blood vessels are dilated, tortuous, and immature, leading to excessive permeability and increased hypoxia [80]. In addition, vascular disorganization causes heterogeneity in the tumor blood vessel network, creating highly vascularized tumor areas and other hypoxic areas with low vascular density [47]. Thus, hypoxia becomes a major driver of tumor angiogenesis, along with other mechanisms promoted by activated oncogenes or loss of tumor suppressor genes, in which lncRNAs play an important role, mainly through acting as competing endogenous RNAs for miRNAs [81]. Similarly, circRNAs have been extensively studied in cancer, elucidating important roles in tumor development, growth, and angiogenesis [82]. For instance, VEGFR-related pathways have been linked to circRNAs by affecting tumor angiogenesis by sponging miRNAs [83][84][83,84]. The landscape of exosomal lnc- and circRNAs in angiogenesis in cancer is summarized in Table 1 and Figure 4.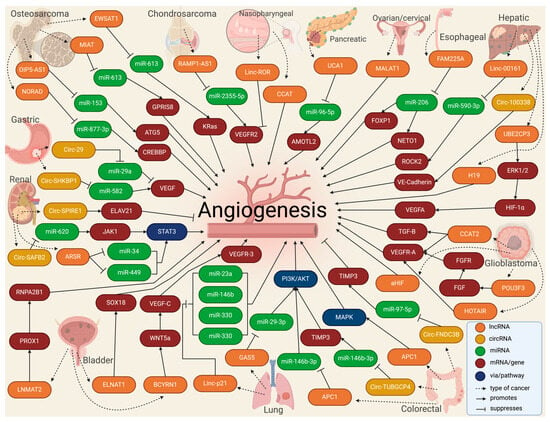
Figure 4.
Molecular landscape of exosomal lnc- and circRNAs in angiogenesis in cancer.
Table 1.
The landscape of exosomal lnc- and circRNAs in angiogenesis in cancer.
| Lnc/circRNA | Molecular Target | Donor Cells | Recipient Cells | Effect | Reference |
|---|---|---|---|---|---|
| OSTEOSARCOMA | |||||
| OIP5-AS1 | miR-153/ATG5 | HOS | HUVECs | Promotes | Li 2021 [85] |
| MIAT | miR-613/GPR158 | U2OS, MG63, and 293T | HUVECs | Promotes | Wang 2022 [86] |
| NORAD | miR-877-3p/CREBBP | 143B, MG-63, Saos2, HOS, and U20S | Osteosarcoma cells | Promotes | Feng 2022 [87] |
| EWSAT1 | miR-326/KRas | 143B, MNNG/HOS, MG63, U20S | BMSCs, HMEC-1 | Promotes | Tao 2020 [88] |
| CHONDROSARCOMA | |||||
| RAMP2-AS1 | miR-2355-5p/VEGFR2 | SW1353 | HUVECs | Promotes | Cheng 2020 [89] |
| PANCREATIC | |||||
| UCA1 | miR-96-5p/AMOTL2 | PANC-1, MIA PaCa-2, BxPC-3, Aspc-1, Sw1990 | HUVECs, HEK293T | Promotes | Guo 2020 [90] |
| NASOPHARYNGEAL | |||||
| Linc-ROR | p-AKT/p-VEGFR2 pathway | CNE2 | HUVECs | Promotes | Zhang 2022 [91] |
| CCAT2 | NR | CNE2, NP69 | HUVECs | Promotes | Zhou 2020 [92] |
| ESOPHAGEAL | |||||
| FAM225A | miR-206/NETO2 and FOXP1 | ECA109, TE-1, KYSE150, KYSE140 | HET-1A, HUVECs | Promotes | Zhang 2020 [93] |
| GLIOMA/GLIOBLASTOMA | |||||
| CCAT2 | VEGF-A and TGF-B | A172, U87-MG, U251, T98G | HUVECs | Promotes | Lang 2017 [94] |
| POU3F3 | bFGF/bFGFR/VEGFA | A172, U87-MG, U251, T98G | HBMECs | Promotes | Lang 2017 [95] |
| HOTAIR | VEGFA | A172 | HBMVECs | Promotes | Ma 2017 [96] |
| aHIF | NR * | U87-MG, U251-MG, A172, T98G | HUVECs | Promotes | Dai 2019 [97] |
| COLORECTAL | |||||
| CircFNDC3B | miR-97-5p/TIMP3 | LoVo, SW480, SW602, HCT116 | HUVECs | Suppresses | Zeng 2020 [98] |
| APC1 | MAPK pathway | HTC116, DLD-1, SW480, LoVo, SW116 | HEK293T, HUVECs | Promotes | Wang 2019 [99] |
| CircTUBGCP4 | miR-146b-3p/PDK2/Akt | SW480 | HEK297T | Promotes | Chen 2023 [100] |
| LIVER/HEPATOCELLULAR | |||||
| LINC00161 | miR-590-3p/ROCK2 axis | Huh-7, HCCLM3, MHCC-97L, MHCC-97H | WRL-68, HUVECs | Promotes | You 2021 [101] |
| UBE2CP3 | ERK1/2/HIF-1α/VEGFA | HepG2, SMMC-7721 | HUVECs | Promotes | Lin 2018 [102] |
| H19 | NR * | Huh-7, Sk-Hep | HUVECs | Promotes | Conigliaro 2015 [103] |
| Circ100338 | VE-Cadherin | Hep3B, HLE, Huh-7, BEL7402, SMCC7721, MHCC97L, HCCLM3, MHCC97H, HCCLM6 | HUVECs | Promotes | Huang 2020 [104] |
| LUNG | |||||
| MFI2-AS1 | miR-107/PI3K/AKT pathway | PC9, A549, H1299 | HUVECs | Promotes | Xu 2023 [105] |
| LincRNA-p21 | miR-23a, miR-146b, miR-330, and miR-494 | H23, HCC44 | HUVECs | Promotes | Castellano 2020 [106] |
| GAS5 | miR-29-3p/PI3K/Akt | 16HBE, A549, H1299, 95D | HUVECs | Promotes | Cheng 2019 [107] |
| RENAL CELL | |||||
| ARSR | miR-34 and miR-449 to upregulate STAT3 pathway | Caki-1, ACHN, 786-O | NR * | Promotes | Zheng 2022 [108] |
| CircSAFB2 | miR-620/JAK1/STAT3 axis | A498, 786-O, Caki-1, Caki-2, 769-P, ACHN | THP-1 | Promotes | Huang 2022 [109] |
| CircSPIRE1 | ELAVL1 protein | NR * | NR * | Suppresses | Shu 2023 [110] |
| BLADDER | |||||
| BCYRN1 | WNT5a/VEGF-C/VEGFR3 | T24, 5637, SVHUC-1 | HLECs, HDLECs, HUVECs | Promotes | Zheng 2021 [111] |
| LNMAT2 | PROX1/RNPA2B1/H3K4 | UM-UC-3, 5637, T24 | HLEC, SV-HUC-1 | Promotes | Chen 2020 [112] |
| ELNAT1 | SOX18 | UM-UC-1, RT112, RT4, UM-UC-3, T24, 5637 | HLEC, SV-HUC-1 | Promotes | Chen 2021 [113] |
| GASTRIC | |||||
| Circ0001190 | miR-587/SOSTDC1 | NR * | NR * | Suppresses | Liu 2022 [114] |
| Circ29 | miR-29a/VEGF pathway | SGC-7901, MGC-803 | HUVECs, HEK297T | Suppresses | Li 2021 [115] |
| CircSHKBP1 | miR-582/HUR/VEGF | AGS, HGC27, BGC823 MGC803, GES1 | HUVECs, HEK293T | Promotes | Xie 2020 [116] |
| CircFCHO2 | miR-194-5p/JAK1/STAT3 pathway | NR * | NR * | Promotes | Zhang 2022 [117] |
| OVARIAN | |||||
| MALAT1 | NR * | SKOV3, HO8910 | SKOV3.ip1, HO8910.PM | Promotes | Qiu 2018 [118] |
| CERVICAL | |||||
| TUG1 | VEGF-A, MMP-9, IL-8 | HeLa, CaSki | HUVECs | Suppresses | Lei 2020 [119] |
| BREAST | |||||
| CircHIPK3 | miR-124-3p/MTDH | NR * | NR * | Promotes | Shi 2022 [120] |
| THYROID | |||||
| FGD5-AS1 | miR-6838-5p/VAV2 axis | SW1736, KAT18 | HUVECs | Promotes | Liu 2022 [121] |
| MULTIPLE MIELOMA | |||||
| CircATP10A | miR-66758-3p, miR-3977, miR-6804-3p, miR-1266-3p, miR-3620-3p | NR * | NR * | Promotes | Yu 2022 [122] |
| ALCOHOL-INDUCED TUMOR | |||||
| HOTAIR and MALAT1 | NR * | NR * | HUVECs, HDMECs | Promotes | Lamichhane 2017 [123] |
| CHOLANGIOCARCINOMA | |||||
| CircCCAC1 | EZH2/SH3GL2 | CCA cells | HUVECs | Promotes | Xu 2021 [124] |
* NR: not reported.
