The global statistics of bone disorders, skeletal defects, and fractures are frightening. Several therapeutic strategies are being used to fix them; however, RNAi-based siRNA therapy is starting to prove to be a promising approach for the prevention of bone disorders because of its advanced capabilities to deliver siRNA or siRNA drug conjugate to the target tissue. Despite its ‘bench-to-bedside’ usefulness and approval by food and drug administration for five siRNA-based therapeutic medicines: Patisiran, Vutrisiran, Inclisiran, Lumasiran, and Givosiran, its use for the other diseases still remains to be resolved. By correcting the complications and complexities involved in siRNA delivery for its sustained release, better absorption, and toxicity-free activity, siRNA therapy can be harnessed as an experimental tool for the prevention of complex and undruggable diseases with a personalized medicine approach.
- RNAi
- siRNA therapy
- bone disorders
- siRNA delivery
1. Introduction
23. Therapeutic Interventions of siRNA in Major Bone Disorders
2.1. Small Interfering RNA Therapy for Osteoporosis
3.1. Small Interfering RNA Therapy for Osteoporosis
Osteoporosis is a systemic skeletal disease manifested as weak bones due to low bone mass and the degraded microstructure of the bone tissue. The epidemiological statistics of osteoporosis are frightening, as every third second there is an osteoporosis-related fracture occurring somewhere in the world. More than 8.9 million fractures are reported annually, and their impact is so severe that an individual comes to know that he/she is suffering from osteoporosis after his/her first fall/fracture [14]. In the face of the advancing challenge to prevent and manage osteoporosis, siRNA therapy has started showing effective results. A few years ago, siRNA was designed to target the inhibition of receptor activator of nuclear factor kappa B (NF-kβ; RANK) to block osteoclastogenesis for the prevention of osteoporosis. This siRNA was delivered by a novel method of delivery known as biocompatible mesoporous bioactive glass (MBG) to the osteoclast cells. The efficiency of siRNA-RANK-MBG to inhibit osteoclastogenesis was observed to be 70 percent, along with significant attenuation of the gene expression of C-Fos, Cathepsin-k (CTSK), Nuclear factor of activated T cells cytoplasmic 1 (NFATc1), and Tartrate-resistant acid phosphatase (TRAP) genes [15]. Another study showed siRNA therapy against heat shock protein 90 (HSP90), which is a significant player in glucocorticoid-induced osteoporosis (GIOP) [16]. siRNA conjugated with 17-Demethoxy-17-allyaminogeldassmyin (17-AAG), which is an inhibitor of HSP90, was used, which significantly reduced the transcriptional output of glucocorticoid signaling, whereas overexpression of HSP90α and HSP90β augmented glucocorticoid expression in calvarial osteoblasts. This finding suggests that bone formation can be preserved by targeting HSP90 with siRNA-17-AAG in GIOP. It is known that Casein kinase 2 interacting protein (CKIP-1) is a negative regulator of bone formation, irrespective of bone resorption. CKIP-1-siRNA was designed, and its effect on osteogenic differentiation, bone mineralization, and bone mass parameters was observed for osteoporosis intervention [17]. The results were encouraging, as the silencing of CKIP-1 mRNA expression increased bone formation significantly without inducing any autoimmune response. Similarly, the sclerostin (SOST) gene, which downregulates Wingless-related integration site (Wnt) signaling and suppresses osteoblast differentiation, was targeted with mesoporous silica nanoparticles (MSNs) coated with SOST-siRNA. It increased ostegenic marker expression and, hence, showed positive results for the prevention of bone mass loss in osteoporosis [18]. The CTSK gene is implicated in bone degradation because of its predominant role in enhancing osteoclastogenesis. To silence its expression and preserve bone mass in osteoporosis, siRNA was designed with an ultrasound-responsive nanodrop, encapsulated with CTSK, and embedded with alendronate (AL) for bone osteoclasts (CTSKsiRNA-ND-AL). This therapy suppressed osteoclastogenesis significantly, highlighting a novel strategy of ultrasound-responsive targeted nanodroplet processes for both gene and drug delivery [19]. Crk proto-oncogene (CrkII), an adaptor protein, plays a key role in cytoskeletal reorganization, phagocytotic cup formation, mitogenesis, osteoclast differentiation, and osteoblast differentiation. CrkII expression promotes c-jun-N-terminal kinase (JNK) phosphorylation and slows down osteoblast differentiation. Moreover, its overexpression reduces bone mass; hence, it is considered a negative regulator of bone remodeling. CrkII siRNA was encapsulated in a bone-targeting peptide (AspSerSer)6-liposome to target receptor activator of nuclear factor kappa-B ligand (RANKL) signaling to improve bone mass in vitro [20].2.2. Small Interfering RNA Therapy for Osteoarthritis
3.2. Small Interfering RNA Therapy for Osteoarthritis
Knee osteoarthritis is a painful joint disorder that is irreversible, causing deformity by degrading articular cartilage, leading to pain and joint dysfunction. It has been revealed that infusion of adipose-derived stem cells (ADSCs) into the knee joint cavity may improve the repair and healing process in knee osteoarthritis. Gene expression profiling of ADSCs in synovial fluid has suggested that they can activate several genes. To understand the role of gene expression for cell viability, siRNA was designed to target FOS like 1, the AP-1 Transcription Factor subunit (FOSL1), and was infused in ADSCs, which showed reduced cell viability, suggesting that FOSL1 is responsible for ADSCs survival in synovial fluid in knee osteoarthritis [21]. This study recommended that for better outcomes in knee osteoarthritis, cultured ADSC-induced therapy should be supplemented with upregulation of FOSL1 expression. High mobility group box chromosomal protein 1 (HMGB-1) plays a significant role in oxidation, inflammation, and apoptosis within chondrocytes, contributing to the development of osteoarthritis. HMGB siRNA was designed and delivered to chondrocytes of knee osteoarthritic patients, which revealed that silencing HMGB-1 mRNA expression significantly attenuated the inflammatory signaling by mediating the effects of matrix metallopeptidase 13 (MMP13), Collagenase, Interleukin-6 (IL-6), and Collagen type II alpha 1 (COL2A1) genes [22]. To understand the effect of nitric oxide (NO) in inflammatory cells, photothermal-triggered NO nanogenerators, NO-Hb@siRNA@PLGA-PEG (NHsPP), were designed for the prevention of osteoarthritis. In this case, hemoglobin (Hb) acted as a NO carrier, which absorbed infrared light (650 nm) and converted it into heat to generate NO. This assembly was loaded with Notch 1-siRNA, which exerted therapeutic effects by inhibiting the synthesis of proinflammatory cytokines and macrophages [23]. This therapy has suggested a novel non-invasive photothermal nanoparticle-based NO-releasing technology to manage inflammatory signaling therapy in osteoarthritis. Anti-inflammatory and immunosuppressive Interleukin 37 (IL-37) plays a role in converting proinflammatory macrophage 1 (M1) to anti-inflammatory M2 during inflammation. To have beneficial effects of IL-37, siRNA-IL-1R8 and MCC-950 were designed and delivered, which exhibited that IL-37 inhibited the expression of Nucleotide-binding domain, leucine-rich, pyrin domain-containing-3 (NLRP3) inflammasome-derived release of proinflammatory cytokines IL-18 and IL-1β along with upregulation of IL-1R8 expression [24]. This inference has suggested the utility and usefulness of siRNA IL-1R8 to suppress inflammasome-based inflammatory cascades in bone disorders. One may imagine that the application of several proven siRNAs may reduce the inflammatory episodes, reverse articular cartilage degradation, and relieve pain in knee osteoarthritis (Figure 1).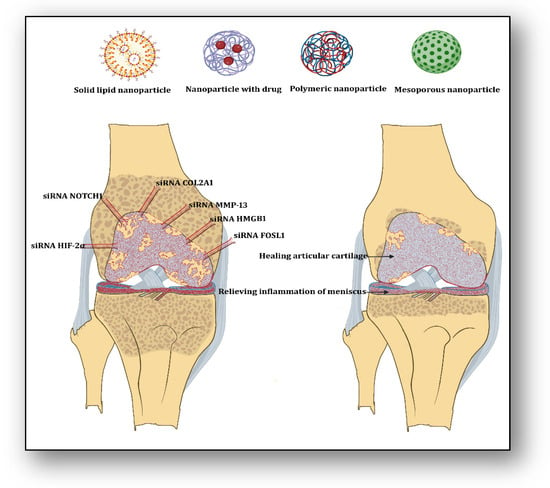
2.3. Small Interfering RNA Therapy for Rheumatoid Arthritis
3.3. Small Interfering RNA Therapy for Rheumatoid Arthritis
2.4. Small Interfering RNA Therapy for Intervertebral Degenerative Disc Disease
3.4. Small Interfering RNA Therapy for Intervertebral Degenerative Disc Disease
Intervertebral degenerative disk disease (IVDD) is an age-related medical condition where one or more intervertebral discs of the spine undergo degeneration, causing severe neck and back pain. It is a multifactorial disease, but primarily its cause is the loss of soluble proteins from the nucleus pulposus (NP) of the intervertebral discs. NP consists of collagen fibrils and plays a significant role in tissue’s generation and maintenance [28]. One of the major causes of IVDD pathology is the apoptosis of NP cells and the continuous degeneration of the extracellular matrix (ECM). It has been observed that A disintegrin and metalloproteinase with thrombospondin motif 5 (ADAMTS5) and Caspase (Cas3) play significant roles in the deterioration of the ECM and apoptosis, respectively. Dual siRNAs against ADAMTS5 and Cas3 were designed, and in vitro analysis revealed that ATS5-Cas3-siRNA increased the regeneration of ECM in damaged discs and reduced apoptosis of NP cells [29]. The results of this study have suggested that such dual siRNA therapy can be a better way to treat IVDD. Sirtuin 1 (SIRT1) is an NAD-dependent deacetylase that slows down apoptosis in many cells. To understand its role in apoptosis of disc NP cells, siRNA against SIRT1 mRNA was designed and delivered in patients with lumbar disc degenerative disease (LDDD) and lumbar vertebral fracture (LVF) [30]. The results revealed that phosphorylation of AKT serine/threonine kinase 1 (AKT) was significantly reduced in NP cells. The results of this study suggest that SIRT1 plays a significant role in NP cell survival via the AKT anti-apoptotic signaling pathway. Another in vitro study investigated the role of Survivin in LDDD and LVF [31]. Survivin plays a crucial role in the regulation of cell division and in the inhibition of apoptosis in NP cells by blocking Caspase activity. siRNA targeting Survivin was designed and investigated in NP cells of LPDD and LVF patients. The results revealed that Survivin siRNA significantly reduced the proliferation rate of NP cells and increased sensitivity to pro-apoptotic signals. This suggests that Survivin plays a fundamental role in the proliferation and prevention of apoptosis of damaged NP cells, leading to inhibition of disc degenerative episodes. In IVDD, low back pain is the main problem, which is enhanced due to the expression of proinflammatory cytokines such as IL-1β, which are abundantly present in NP cells. SIRT1 supports the regulation of aging and immune responses through anti-apoptotic and anti-catabolic signaling. siRNA against the SIRT1 gene was designed, and its effect was investigated in NP cells, which revealed that SIRT1 siRNA increased IL-1β-induced apoptosis and enhanced the expression of MMPs [32]. NF-kB p65 plays an important role in IVDD pathology. It was observed that mRNA levels of the trigger receptor expressed on myeloid cells-2 (TREM2) are strongly correlated to NF-kBp65 in the NP cells of IVDD patients. TREM2-siRNA was designed and transfected into NP cells, which revealed that it significantly reduced cell apoptosis, enhanced cell proliferation, suppressed proinflammatory cytokines (IL-1β, IL-6, and TNF-α), and downregulated NF-kBp65 expression in NP cells [33].2.5. Small Interfering RNA Therapy for Fracture Healing
3.5. Small Interfering RNA Therapy for Fracture Healing
Fractures due to bone disorders impact substantially skeletal health globally. Worldwide, around 178 million fractures have been observed in 2019, with a future upward trend [1]. Approximately one-tenth of these fractures are either delayed or remain non-union. In the clinical arena of orthopedics, less efficient pharmacological strategies are available for the healing and regeneration of bone fractures. Hence, novel mechanisms are required to accelerate the repair and regeneration of fractures. Some studies have suggested that the plasminogen activation system plays a significant role in bone remodeling and metabolism by removing fibrin and excessive plasmin-associated fibrinolysis [34][35][36]. A siRNA-based study has investigated the role of Plasminogen activator inhibitor-2 (SerpinB2) in fracture healing and repair [37]. SerpinB2 is a serine protease that inactivates tissue plasminogen activator in human bone marrow mesenchymal stem cells (hBMSCs). siRNA was designed to target the SerpinB2 gene and delivered to hBMSCs, which revealed that silencing SerpinB2 significantly increased osteoblast differentiation of hBMSCs and mineralization in vitro and increased β-catenin levels. It was observed and validated that injection of SerpinB2 siRNA locally into the tibial fracture improved fracture healing. The results suggest that SerpinB2 siRNA can effectively promote fracture healing in vivo, making it a potent target for clinical management of fractures. Another study utilized low-intensity pulsed ultrasound (LIPUS) to investigate its fracture healing capabilities mediated by the Hippo signaling pathway [38]. Key transcriptional co-activators of the Hippo pathway are Yes-associated protein1 (YAP) and Transcriptional co-activator with PDZ-binding domain (TAZ), which were knocked down by siRNA and shRNA, respectively, to investigate whether LIPUS can activate YAP/TAZ expression. The results revealed that both of these angiogenic and vascular remodelers were significantly increased after LIPUS treatment. The results of this study have proposed that LIPUS treatment mediates the Hippo pathway to angiogenesis-mediated fracture healing. The Chordin gene (CHRD) is a pleiotropic gene that encodes the Chordin protein that regulates dorsal-ventral tissue differentiation in early embryonic development. Chordin was observed to be highly expressive in fractures.34. Mechanisms for siRNA Delivery in Bone Disorders
3.1. Cationic Polymer-Based Delivery Systems
4.1. Cationic Polymer-Based Delivery Systems
Organic polymers, which are cationic in nature, demonstrate characteristics such as lowimmunogenicity and functional groups for essential interactions with cellular components. When cationic polymers are conjugated with biomolecules such as siRNA, these act as nanocarriers for their effective and efficient delivery to the target tissue for their salubrious effects. The earliest cationic polymer-mediated siRNA delivery was reported by Bologna et al. in 2003, in which polyethyleneimine (PEI) was complexed with P2X3 siRNA for its delivery to the hamster ovary cells for the successful knockdown of the P2X3 gene in the ovary cells [39]. The positive charge on these polymers stabilizes the highly negative charge on siRNA, which increases its uptake and prolongs its persistence in the target tissue, evades its lysis by nucleases, and helps in their endolysosomal escape [40]. Cationic polymer-based delivery systems include cationic polymer-derived nanoparticles, spermine-based delivery, Chitosan-mediated delivery, Atelocollagen-mediated delivery, and Albumin-based nanoparticles.34.1.1. Cationic Polymer-Derived Nanoparticles
In this technique, synthetic biopolymers such as Polylactic-co-glycolic acid (PLGA) are combined with cationic polymers such as PEI to form cationic polymer-derived nanoparticles. PLGA has various attributes, such as biocompatibility, biodegradability, low immunogenicity, and high stability in body fluids. PEI, being cationic, can form non-covalent linkages with highly negative biomolecules such as siRNA, which can lead to their efficient delivery to the target tissue by protecting them from lysis by nucleases, facilitating their tissue internalization, and facilitating their endolysosomal escape to the cytosol [41]. Cytotoxicity and genotoxic effects are noticed when a high concentration of PEI is used due to its high charge density, which can be reduced by its acetylation (Ac), making it safe as a vector for siRNA delivery. An acetylated PLGA-PEI (Ac-PLGA-PEI) nanocomplexgel was designed to deliver Matrix metalloproteinase 2 (MMP-2) siRNA to the PC3 cell line of a human prostate tumor for gene silencing and to the collagen matrix embedded in the human chondrocyte cell line C20A4 for chondrocyte dedifferentiation (a hallmark process of osteoarthritis) in vitro to develop a siRNA therapy for osteoarthritis. PC3 cells and C20A4 cells exhibited efficient cellular uptake of MMP-2 siRNA, effective endosome escape, and successful knockdown of MMP-2 expression, coupled with inhibition of chondrocytic dedifferentiation-related genes, collagen type II alpha 1 chain (COL2A1) and Aggrecan (ACAN). It also prevented matrix breakdown in C20A4 cells when transfected with the MMP-2 siRNA/nanogel complex [41]. RANK siRNA-conjugated PLGA nanoparticles can be embedded into bone augmentation biomaterials such as calcium phosphate cements (CPC), which improve the anchoring of implants around weak osteoporotic bones and allow for early mobilization, which accelerates fracture healing (22951320) [42].34.1.2. Spermine-Based Delivery System
Spermine is an endogenous polyamine that is commonly found in human sperm. In the physiological condition, spermine contains a positive charge, which helps stabilize deoxyribonucleic acid (DNA), leading to its condensation in the sperm. Spermine is a cationic polymer that demonstrates characteristics such as biocompatibility, negligible toxicity, and biodegradability. Due to these properties, Spermine is able to conjugate with different groups of polymers to form Polyspermine-based nanocarriers to transport various types of biomolecules, such as siRNA and drugs, to the target tissue [43]. A polyspermine imidazole-4,5-imine (PSI) polyplex was developed by conjugating Chordin siRNA with PSI to deliver Chordin siRNA to the bone-forming mesenchymal stem cells (MSCs) extracted from patients suffering from bone nonunion fracturesin vitro and to the tibial monocortical defect modelin vivo.34.1.3. Chitosan-Mediated Delivery System
Chitosan is a cationic polysaccharide formed by the deacetylation of chitin. Being structurally similar to chitin, a primary component of the arthropod exoskeleton, chitosan is known for its low cytotoxicity, biocompatibility, and biodegradability. Chitosan has the ability to combine with drugs and biomolecules like siRNA, which facilitates their endosomal escape to the cytoplasm in the cells and avoids their nuclease-mediated lysis, hence helping in their direct delivery to the target tissue. Chitosan has poor solubility at physiological pH, which can be resolved by binding it to molecules that increase its bio-solubility [44]. A folic acid-coupled novel polysaccharide derivative formed by azidized chitosan conjugated with poly (L-lysine) dendrons (PLLD) was created for the allocation of astrocyte elevated gene-1 (AEG-1) siRNA to the 143B and U20S cell lines of human osteosarcoma in vitro.34.1.4. Atelocollagen-Mediated Delivery System
Atelocollagen is a highly purified pepsin-treated Type-I collagen that is derived from the calf dermis. Atelocollagen has low immunogenicity because of its telopeptide-free structure. Due to its similarity with the most commonly found protein in the body, i.e., collagen, Atelocollagen exhibits low cytotoxicity, extreme biocompatibility, and is easily biodegradable [45]. When combined with biomolecules such as siRNA, Atelocollagen supports its effective cellular uptake, resistance to nucleases, and prolonged release of siRNA to the target tissue.34.1.5. Albumin-Based Nanoparticles
Human serum albumin (HSA) is a water-soluble globular protein that is abundantly present in human plasma. HSA is rich in cysteine residues that are acidic, hence cationic in nature, and form sulfhydryl linkages to bind its three peptide chains for maintaining its tertiary conformation. Moreover, the surface of HSA is rich in these cysteine residues, which participate prominently in the conjugation of a variety of biomolecules, such as siRNA, to HSA molecules. Due to these structural properties, HSA functions as an extraordinary carrier protein in physiological conditions to supply nutrients to the body tissues [46].3.2. Lipid-Based Delivery Systems
4.2. Lipid-Based Delivery Systems
Lipids, one of the most multifaceted molecules, are commonly used as drug carriers for curing bone diseases. The amphiphilic behavior of lipids contributes to the formation of micelles and layers, which can encapsulate biomolecules like siRNA (lipoplexes), hence can be used as vehicles for their direct delivery to the target tissue [47]. The earliest use of lipids as a delivery vehicle for siRNA to the target tissue was reported by Sorenson et al. (2003), in which siRNA was delivered to silence TNF-α using 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) cationic liposomes to murine peritoneal macrophages in vitro and to the Bagg Albino (BALB/c) mouse model in vivo, which resulted in the suppression of TNF-α gene expression in both murine macrophages in vitro and in vivo [48]. Lipid-delivery systems include nanomicelles and liposomes.34.2.1. Nanomicelles-Based Delivery System
Nanomicelles are colloidal structures composed of amphiphilic monomers with a small hydrophobic head, which lines the interior of the micelle, and a long hydrophilic tail, which makes the exterior of the micelle in an aqueous medium [49]. When complexed with different types of polymers, nanomicelles act as carriers for transporting siRNA to the target bone tissue. A bio-mimetic system was formulated by combining lipoic acid (LA) with cross-linked peptide-lipoic acid micelle (LACL) to deliver sterol regulatory element-binding protein-1 (SREBP1) siRNA and docetaxel (DTX) to the cell lines PC-3 and C4-2B of human bone metastatic castration-resistant prostate cancer (BmCRPC) in vitro. Effective silencing of SREBP-1 and stearoyl Co-A desaturase 1 (SCD-1; down regulatory gene of SBREP1), along with increased uptake of siRNA by the tumor cells, combinatorial attack on bone cancer, and anti-proliferating and anti-invasive effects of both DTX and siRNA, were observed in the two cell typesin vitro.34.2.2. Liposomes-Based Delivery Systems
Liposomes are globular lipid vesicles formed from one or more lipid layers as a result of the emulsification of lipids in an aqueous medium [50]. Liposomes are a multifaceted delivery vehicle for a variety of drugs and biomolecules, such as siRNA, because of their similar composition to the membranes of cells and organelles, which aid in direct delivery to the target site. Doxil was the first FDA-approved nano-drug based on a cationic liposome-mediated delivery system for treating a variety of cancers [51]. Liposomes are composed of different types of lipids (both natural and synthetic) and can be complexed with different types of cationic polymers and cationic peptides. This makes them an excellent nano-delivery system to transport siRNA into the targeted bone tissue, as they can prompt their efficient penetration into the cells and nuclear localization without causing auto-immunity. It entraps siRNA within them by encapsulation to avoid interaction with serum proteins. A cationic liposome-transfecting agent, LipofectamineTM 3000, was fused with high-temperature serine protease A (HtrA2) siRNA into the fibroblast-like synoviocytes (FLSs) derived from individuals suffering from rheumatoid arthritis and osteoarthritis with articular replacement surgery in vitro. Suppression of the HtrA2 gene along with inhibition of expression of various pro-inflammatory biomarkers like IL-1β, TNF-α, CCL-2, IL-6, and IL-8 was observed without exhibiting any cytotoxicity in the FLS cells in vitro [52].3.3. Aptamer-Based Delivery System
4.3. Aptamer-Based Delivery System
Aptamers are a type of nucleic acid sequence that is generally composed of a single-stranded RNA or DNA that is 25–30 nt bases long. Aptamers are arranged in a distinct tertiary conformation and conjugated to a variety of nanoparticles to design an efficient non-viral vector system for drug delivery [53]. These aptamer-modified nanoparticles can recognize and bind to their target molecule, such as siRNA, with high specificity and affinity, thereby facilitating their nuclear localization and enhanced cellular uptake by the target cells. An anti-ROS osteoblast-specific, CH-6 aptamer-modified manganese ferrite nanoparticle system was constructed to deliver bone morphogenetic protein-2 (BMP2) siRNA to human mesenchymal stem cells (hMSCs)in vitro.3.4. Inorganic-Based Delivery Systems
4.4. Inorganic-Based Delivery Systems
34.4.1. Titanium Implant-Based Delivery System
Titanium oxide is an excellent implant material because of its biocompatibility, corrosive resistance, and low elasticity. The titanium surface forms attachment to the bones via a process called osseointegration, in which biomolecules are absorbed on the titanium oxide layer along with the migration of osteoblasts and other immune cells into the implant [54]. This process of osseointegration makes titanium implants an outstanding vehicle to transport biomolecules and drugs to their target sites. When these titanium implants are embedded with nanopolymers or nanoparticles encapsulating siRNA, they can be used as carriers for siRNA in the target bone tissue. A titanium implant coated with a multi-layered film made of sodium hyaluronate and chitosan nanoparticles encapsulating siRNA by a layer-by-layer (LbL) process was constructed to deliver green fluorescent protein (GFP) siRNA and casein kinase-2 interacting protein-1 (Ckip-1) siRNA to the H1299 cell line of human lung carcinoma and the MG63 human cell line of osteosarcoma, respectively, in vitro.34.4.2. Iron Oxide Nanocage-Based System
Iron Oxide (IO) nanoparticles are spherical cage-like structures that are composed of magnetite (Fe3O4) and maghemite (γ-Fe3O4) forms of iron oxide, which are supramagnetic in nature. These IO-nanocages are chemically inert and also exhibit properties like strong magnetic and catalytic behavior, biocompatibility with low cytoxicity, and high stability in biological fluids, which makes them exceptional vectors for drug/biomolecule (siRNA) delivery to the required tissues, such as bone [55]. When placed under an alternating magnetic field, IO-nanocages exhibit Brownian motion, which helps in their endosomal escape and direct delivery of siRNA into the cytoplasm of the target cell, facilitating their efficient delivery [56].34.4.3. Cerium Oxide Nanoparticles
Cerium oxide nanoparticles (CeNPs), also known as nanoceria, have a cubical structure consisting of cerium ions existing in +3 and +4 states, which contribute totheir anti-oxidant properties that mimic superoxide dismutase (SOD) and phosphatase enzyme [57]. Moreover, cerium oxide (CeO2) exhibits ferromagnetic behavior and is non-toxic to living cells. When complexed with different biomolecules, such as siRNA, via encapsulation within their lattice, CeNPs can be employed as nanocarriers for the optimal delivery of these molecules to the target bone tissue. An ultrasound-mediated nanobubble delivery system was engineered, for which nanobubbles were created from cerium oxide nanoparticles (CeNPs) complexed with albumin and perfluorohexane. These were loaded with CTSK siRNA for its delivery to the human bone-marrow-derived mesenchymal stem cells (hMSCs) for gene silencing and to the human osteoclast precursors for bone regeneration invitro.34.4.4. Silica-Based Nanoparticles
Porous silicon is procured by an electrochemical etching of monocrystalline silicon in hydrofluoric acid. Silica, also known as silicon dioxide, is obtained from porous silicon, which is known for its physiochemical properties such as tunable pore size, porosity, biocompatibility, bioinert behavior, and biodegradability [58]. Porous silica nanoparticles facilitate the bioavailability and persistence of the drug for a longer period of time, reduce the toxicity of the drug, and provide precise drug targeting [58]. Because of these characteristics, when combined with a variety of drugs and biomolecules, such as siRNA, porous silica nanoparticles can be exploited as a nanocarrier system for these molecules in the tissue of interest.3.5. Nucleofection-Based Delivery System
4.5. Nucleofection-Based Delivery System
Nucleofection is an electroporation-mediated transfection technique that was invented by the biotechnology company Amaxa. Nucleofection involves the use of a specific voltage generator by a device called a nucleofactor along with cell-specific nucleofection reagents to deliver therapeutic biomolecules such as siRNA directly into the cells of the target tissue. Nucleofection is beneficial over lipid-based transfection due to its non-toxicity towards cell types and non-activation of interferons, and it is best for transfection of difficult-to-transfect cell lines such as primary and non-adherent cell lines.3.6. Quantum Dot-Based Delivery System
4.6. Quantum Dot-Based Delivery System
Quantum dots (QDs) are inorganic semiconductors that are synthesized by using metal ions and colloid stabilizers, whose surfaces can be modulated through shape control, surface coating, and surface functionalization. These properties help in their local targeting and efficient cellular uptake, but QDs exhibit cytotoxicity in higher quantities. When QDs are combined with other polymers, it lowers their toxicity and makes them biocompatible and biodegradable, ultimately establishing QD nanoparticles as efficient nanocarriers for the delivery of drugs and biomolecules such as siRNA to the target bone tissue, which can help in developing therapies for bone defects [59][60].45. A Combinatorial Approach to siRNA Therapy for Various Bone Disorders
A newer strategy, i.e., a combination of chemical drugs and siRNA loaded onto various types of nanoformulations, has been developed as an efficient therapy to treat bone diseases. This dual therapy approach comes with a lot of benefits due to the synergistic effect between drugs and siRNA on the target tissue. The nanoparticle formulations are developed in such a way that they minimize the cytotoxic effects of drugs, off-target silencing via selective accumulation, and suppression of auto-immune reactions in the body due to their non-recognition by TLRs [61]. Similarly, another study reported a dual approach of PEI-modified PLGA nanoparticles to deliver dexamethasone and anti-COX-2 siRNA to the human chondrocyte cell line C28/12, in which rheumatoid arthritis (RA) conditions were induced by TNF-α treatment. Effective silencing of COX-2 expression without any cytotoxicity attenuated expression of pro-inflammatory markers, microsomal prostaglandin E synthase-1(mPGES-1) and inducible nitric oxide synthase (iNOS), along with reduced expression of apoptotic markers, capase-3 and annexin-V [62].References
- GBD 2019 Fracture Collaborators. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592.
- GBD 2017 DALYs and HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922.
- Singh, M.; Singh, B.; Sharma, K.; Kumar, N.; Mastana, S.; Singh, P. A Molecular Troika of Angiogenesis, Coagulopathy and Endothelial Dysfunction in the Pathology of Avascular Necrosis of Femoral Head: A Comprehensive Review. Cells 2023, 12, 2278.
- Ren, Y.; Liu, B.; Feng, Y.; Shu, L.; Cao, X.; Karaplis, A.; Goltzman, D.; Miao, D. Endogenous PTH Deficiency Impairs Fracture Healing and Impedes the Fracture-Healing Efficacy of Exogenous PTH(1-34). PLoS ONE 2011, 6, e23060.
- Lo Sicco, C.; Tasso, R. Harnessing Endogenous Cellular Mechanisms for Bone Repair. Front. Bioeng. Biotechnol. 2017, 5, 52.
- Peng, Y.; Li, J.; Lin, H.; Tian, S.; Liu, S.; Pu, F.; Zhao, L.; Ma, K.; Qing, X.; Shao, Z. Endogenous Repair Theory Enriches Construction Strategies for Orthopaedic Biomaterials: A Narrative Review. Biomater. Transl. 2021, 2, 343–360.
- Chen, Y.G.; Hur, S. Cellular Origins of dsRNA, Their Recognition and Consequences. Nat. Rev. Mol. Cell Biol. 2022, 23, 286–301.
- McCaffrey, A.P.; Meuse, L.; Pham, T.-T.T.; Conklin, D.S.; Hannon, G.J.; Kay, M.A. RNA Interference in Adult Mice. Nature 2002, 418, 38–39.
- Song, E.; Lee, S.-K.; Wang, J.; Ince, N.; Ouyang, N.; Min, J.; Chen, J.; Shankar, P.; Lieberman, J. RNA Interference Targeting Fas Protects Mice from Fulminant Hepatitis. Nat. Med. 2003, 9, 347–351.
- Weng, Y.; Xiao, H.; Zhang, J.; Liang, X.-J.; Huang, Y. RNAi Therapeutic and Its Innovative Biotechnological Evolution. Biotechnol. Adv. 2019, 37, 801–825.
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med. 2019, 380, 549–558.
- Sas, D.J.; Magen, D.; Hayes, W.; Shasha-Lavsky, H.; Michael, M.; Schulte, I.; Sellier-Leclerc, A.-L.; Lu, J.; Seddighzadeh, A.; Habtemariam, B.; et al. Phase 3 Trial of Lumasiran for Primary Hyperoxaluria Type 1: A New RNAi Therapeutic in Infants and Young Children. Genet. Med. 2022, 24, 654–662.
- Schett, G.; Loza, M.J.; Palanichamy, A.; FitzGerald, O.; Ritchlin, C.; Bay-Jensen, A.-C.; Nielsen, S.H.; Gao, S.; Hsia, E.C.; Kollmeier, A.P.; et al. Publisher Correction to: Collagen Turnover Biomarkers Associate with Active Psoriatic Arthritis and Decrease with Guselkumab Treatment in a Phase 3 Clinical Trial (DISCOVER-2). Rheumatol. Ther. 2022, 9, 1247–1248.
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609.
- Kim, T.-H.; Singh, R.K.; Kang, M.S.; Kim, J.-H.; Kim, H.-W. Inhibition of Osteoclastogenesis through siRNA Delivery with Tunable Mesoporous Bioactive Nanocarriers. Acta Biomater. 2016, 29, 352–364.
- Chen, H.; Xing, J.; Hu, X.; Chen, L.; Lv, H.; Xu, C.; Hong, D.; Wu, X. Inhibition of Heat Shock Protein 90 Rescues Glucocorticoid-Induced Bone Loss through Enhancing Bone Formation. J. Steroid Biochem. Mol. Biol. 2017, 171, 236–246.
- Guo, B.; Zhang, B.; Zheng, L.; Tang, T.; Liu, J.; Wu, H.; Yang, Z.; Peng, S.; He, X.; Zhang, H.; et al. Therapeutic RNA Interference Targeting CKIP-1 with a Cross-Species Sequence to Stimulate Bone Formation. Bone 2014, 59, 76–88.
- Mora-Raimundo, P.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Nanoparticles to Knockdown Osteoporosis-Related Gene and Promote Osteogenic Marker Expression for Osteoporosis Treatment. ACS Nano 2019, 13, 5451–5464.
- Shar, A.; Aboutalebianaraki, N.; Misiti, K.; Sip, Y.Y.L.; Zhai, L.; Razavi, M. A Novel Ultrasound-Mediated Nanodroplet-Based Gene Delivery System for Osteoporosis Treatment. Nanomedicine 2022, 41, 102530.
- Seong, S.; Vijayan, V.; Kim, J.H.; Kim, K.; Kim, I.; Cherukula, K.; Park, I.-K.; Kim, N. Nano-Formulations for Bone-Specific Delivery of siRNA for CrkII Silencing-Induced Regulation of Bone Formation and Resorption to Maximize Therapeutic Potential for Bone-Related Diseases. Biomater. Sci. 2023, 11, 2581–2589.
- Kitajima, H.; Sakamoto, T.; Horie, T.; Kuwano, A.; Fuku, A.; Taki, Y.; Nakamura, Y.; Tanida, I.; Sunami, H.; Hirata, H.; et al. Synovial Fluid Derived from Human Knee Osteoarthritis Increases the Viability of Human Adipose-Derived Stem Cells through Upregulation of FOSL1. Cells 2023, 12, 330.
- Zhang, C.; Yu, W.; Huang, C.; Ding, Q.; Liang, C.; Wang, L.; Hou, Z.; Zhang, Z. Chrysin Protects Human Osteoarthritis Chondrocytes by Inhibiting Inflammatory Mediator Expression via HMGB1 Suppression. Mol. Med. Rep. 2019, 19, 1222–1229.
- Chen, X.; Liu, Y.; Wen, Y.; Yu, Q.; Liu, J.; Zhao, Y.; Liu, J.; Ye, G. A Photothermal-Triggered Nitric Oxide Nanogenerator Combined with siRNA for Precise Therapy of Osteoarthritis by Suppressing Macrophage Inflammation. Nanoscale 2019, 11, 6693–6709.
- Luo, P.; Peng, S.; Yan, Y.; Ji, P.; Xu, J. IL-37 Inhibits M1-like Macrophage Activation to Ameliorate Temporomandibular Joint Inflammation through the NLRP3 Pathway. Rheumatology 2020, 59, 3070–3080.
- Chen, D.; Zeng, S.; Huang, M.; Xu, H.; Liang, L.; Yang, X. Role of Protein Arginine Methyltransferase 5 in Inflammation and Migration of Fibroblast-like Synoviocytes in Rheumatoid Arthritis. J. Cell. Mol. Med. 2017, 21, 781–790.
- Zhao, M.; Zhu, T.; Chen, J.; Cui, Y.; Zhang, X.; Lee, R.J.; Sun, F.; Li, Y.; Teng, L. PLGA/PCADK Composite Microspheres Containing Hyaluronic Acid-Chitosan siRNA Nanoparticles: A Rational Design for Rheumatoid Arthritis Therapy. Int. J. Pharm. 2021, 596, 120204.
- Zou, Y.; Shen, C.; Shen, T.; Wang, J.; Zhang, X.; Zhang, Q.; Sun, R.; Dai, L.; Xu, H. LncRNA THRIL Is Involved in the Proliferation, Migration, and Invasion of Rheumatoid Fibroblast-like Synoviocytes. Ann. Transl. Med. 2021, 9, 1368.
- Gilchrist, C.L.; Francisco, A.T.; Plopper, G.E.; Chen, J.; Setton, L.A. Nucleus Pulposus Cell-Matrix Interactions with Laminins. Eur. Cell Mater. 2011, 21, 523–532.
- Banala, R.R.; Vemuri, S.K.; Dar, G.H.; Palanisamy, V.; Penkulinti, M.; Surekha, M.V.; Gurava Reddy, A.V.; Nalam, M.R.; Subbaiah, G. Efficiency of Dual siRNA-Mediated Gene Therapy for Intervertebral Disc Degeneration (IVDD). Spine J. 2019, 19, 896–904.
- Wang, D.; Hu, Z.; Hao, J.; He, B.; Gan, Q.; Zhong, X.; Zhang, X.; Shen, J.; Fang, J.; Jiang, W. SIRT1 Inhibits Apoptosis of Degenerative Human Disc Nucleus Pulposus Cells through Activation of Akt Pathway. Age 2013, 35, 1741–1753.
- Lin, Y.; Yue, B.; Xiang, H.; Liu, Y.; Ma, X.; Chen, B. Survivin Is Expressed in Degenerated Nucleus Pulposus Cells and Is Involved in Proliferation and the Prevention of Apoptosis In Vitro. Mol. Med. Rep. 2016, 13, 1026–1032.
- Shen, J.; Fang, J.; Hao, J.; Zhong, X.; Wang, D.; Ren, H.; Hu, Z. SIRT1 Inhibits the Catabolic Effect of IL-1β Through TLR2/SIRT1/NF-κB Pathway in Human Degenerative Nucleus Pulposus Cells. Pain Physician 2016, 19, E215–E226.
- Bai, M.; Yin, H.-P.; Zhao, J.; Li, Y.; Wu, Y.-M. Roles of TREM2 in Degeneration of Human Nucleus Pulposus Cells via NF-κB P65. J. Cell. Biochem. 2018, 119, 8784–8796.
- Gibson, B.H.Y.; Duvernay, M.T.; Moore-Lotridge, S.N.; Flick, M.J.; Schoenecker, J.G. Plasminogen Activation in the Musculoskeletal Acute Phase Response: Injury, Repair, and Disease. Res. Pract. Thromb. Haemost. 2020, 4, 469–480.
- Wang, L.; Yao, L.; Duan, H.; Yang, F.; Lin, M.; Zhang, R.; He, Z.; Ahn, J.; Fan, Y.; Qin, L.; et al. Plasminogen Regulates Fracture Repair by Promoting the Functions of Periosteal Mesenchymal Progenitors. J. Bone Miner. Res. 2021, 36, 2229–2242.
- Yuasa, M.; Mignemi, N.A.; Nyman, J.S.; Duvall, C.L.; Schwartz, H.S.; Okawa, A.; Yoshii, T.; Bhattacharjee, G.; Zhao, C.; Bible, J.E.; et al. Fibrinolysis Is Essential for Fracture Repair and Prevention of Heterotopic Ossification. J. Clin. Investig. 2015, 125, 3117–3131.
- Hang, K.; Ying, L.; Bai, J.; Wang, Y.; Kuang, Z.; Xue, D.; Pan, Z. Knockdown of SERPINB2 Enhances the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Activation of the Wnt/β-Catenin Signalling Pathway. Stem Cell Res. Ther. 2021, 12, 525.
- Xu, X.-M.; Xu, T.-M.; Wei, Y.-B.; Gao, X.-X.; Sun, J.-C.; Wang, Y.; Kong, Q.-J.; Shi, J.-G. Low-Intensity Pulsed Ultrasound Treatment Accelerates Angiogenesis by Activating YAP/TAZ in Human Umbilical Vein Endothelial Cells. Ultrasound Med. Biol. 2018, 44, 2655–2661.
- Bologna, J.-C.; Dorn, G.; Natt, F.; Weiler, J. Linear Polyethylenimine as a Tool for Comparative Studies of Antisense and Short Double-Stranded RNA Oligonucleotides. Nucleosides Nucleotides Nucleic Acids 2003, 22, 1729–1731.
- Qadir, A.; Gao, Y.; Suryaji, P.; Tian, Y.; Lin, X.; Dang, K.; Jiang, S.; Li, Y.; Miao, Z.; Qian, A. Non-Viral Delivery System and Targeted Bone Disease Therapy. Int. J. Mol. Sci. 2019, 20, 565.
- Conte, R.; Finicelli, M.; Borrone, A.; Margarucci, S.; Peluso, G.; Calarco, A.; Bosetti, M. MMP-2 Silencing through siRNA Loaded Positively-Charged Nanoparticles (AcPEI-NPs) Counteracts Chondrocyte De-Differentiation. Polymers 2023, 15, 1172.
- Wang, Y.; Tran, K.K.; Shen, H.; Grainger, D.W. Selective Local Delivery of RANK siRNA to Bone Phagocytes Using Bone Augmentation Biomaterials. Biomaterials 2012, 33, 8540–8547.
- Peng, L.; Wagner, E. Polymeric Carriers for Nucleic Acid Delivery: Current Designs and Future Directions. Biomacromolecules 2019, 20, 3613–3626.
- Al-Absi, M.Y.; Caprifico, A.E.; Calabrese, G. Chitosan and Its Structural Modifications for siRNA Delivery. Adv. Pharm. Bull. 2023, 13, 275–282.
- Minakuchi, Y.; Takeshita, F.; Kosaka, N.; Sasaki, H.; Yamamoto, Y.; Kouno, M.; Honma, K.; Nagahara, S.; Hanai, K.; Sano, A.; et al. Atelocollagen-Mediated Synthetic Small Interfering RNA Delivery for Effective Gene Silencing in Vitro and in Vivo. Nucleic Acids Res. 2004, 32, e109. Available online: https://pubmed.ncbi.nlm.nih.gov/15272050/ (accessed on 23 November 2023).
- Srivastava, A.; Prajapati, A. Albumin and Functionalized Albumin Nanoparticles: Production Strategies, Characterization, and Target Indications. Asian Biomed. 2020, 14, 217–242. Available online: https://pubmed.ncbi.nlm.nih.gov/37551304/ (accessed on 23 November 2023).
- Zhang, S.; Zhi, D.; Huang, L. Lipid-Based Vectors for siRNA Delivery. J. Drug Target. 2012, 20, 724–735.
- Sørensen, D.R.; Leirdal, M.; Sioud, M. Gene Silencing by Systemic Delivery of Synthetic siRNAs in Adult Mice. J. Mol. Biol. 2003, 327, 761–766.
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, Properties and Applications in Drug Delivery. IET Nanobiotechnol. 2021, 15, 19–27.
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. Available online: https://pubmed.ncbi.nlm.nih.gov/35600452/ (accessed on 23 November 2023).
- Barenholz, Y. Doxil®—the First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134.
- Jeong, G.H.; Nam, M.K.; Hur, W.; Heo, S.; Lee, S.; Choi, E.; Park, J.H.; Park, Y.; Kim, W.U.; Rhim, H.; et al. Role of High-Temperature Requirement Serine Protease A 2 in Rheumatoid Inflammation. Arthritis Res. Ther. 2023, 25, 96. Available online: https://pubmed.ncbi.nlm.nih.gov/37287073/ (accessed on 23 November 2023).
- Liu, X. Bone Site-Specific Delivery of siRNA. J. Biomed. Res. 2016, 30, 264–271.
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2021, 12, 47.
- Vallabani, N.V.S.; Singh, S. Recent Advances and Future Prospects of Iron Oxide Nanoparticles in Biomedicine and Diagnostics. 3 Biotech 2018, 8, 279.
- Kang, M.A.; Fang, J.; Paragodaarachchi, A.; Kodama, K.; Yakobashvili, D.; Ichiyanagi, Y.; Matsui, H. Magnetically Induced Brownian Motion of Iron Oxide Nanocages in Alternating Magnetic Fields and Their Application for Efficient siRNA Delivery. Nano Lett. 2022, 22, 8852–8859.
- Singh, K.R.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium Oxide Nanoparticles: Properties, Biosynthesis and Biomedical Application. RSC Adv. 2020, 10, 27194–27214.
- Betty, C.A. Porous Silicon: A Resourceful Material for Nanotechnology. Recent Pat. Nanotechnol. 2008, 2, 128–136.
- Obonyo, O.; Fisher, E.; Edwards, M.; Douroumis, D. Quantum Dots Synthesis and Biological Applications as Imaging and Drug Delivery Systems. Crit. Rev. Biotechnol. 2010, 30, 283–301.
- Yukawa, H.; Sato, K.; Baba, Y. Theranostics Applications of Quantum Dots in Regenerative Medicine, Cancer Medicine, and Infectious Diseases. Adv. Drug Deliv. Rev. 2023, 200, 114863.
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345.
- Park, J.S.; Yang, H.N.; Jeon, S.Y.; Woo, D.G.; Kim, M.S.; Park, K.-H. The Use of Anti-COX2 siRNA Coated onto PLGA Nanoparticles Loading Dexamethasone in the Treatment of Rheumatoid Arthritis. Biomaterials 2012, 33, 8600–8612.
