You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Muhammad Sajid and Version 2 by Lindsay Dong.
Plants, being sessile, have developed complex signaling and response mechanisms to cope with biotic and abiotic stressors. Jasmonic acid (JA) and its derivatives, collectively referred to as jasmonates (JAs), are of particular importance and are involved in diverse signal transduction pathways to regulate various physiological and molecular processes in plants, thus protecting plants from the lethal impacts of abiotic and biotic stressors. Jasmonic acid has emerged as a central player in plant defense against biotic stress and in alleviating multiple abiotic stressors in plants, such as drought, salinity, vernalization, and heavy metal exposure.
- abiotic stresses
- biosynthesis
- jasmonic acid
- Jasmonates
- plant hormones
- plant growth
- signaling
1. Introduction
Plant growth and development are profoundly affected by both biotic and abiotic stress factors. The abiotic factors include drought, salinity, water logging, heat, freezing, and heavy metal stress [1][2][3][4]. Drought stress causes osmotic stress which results in dehydration of plant cells leading to plant death [4]. Salinity is also very lethal for the osmoregulation system in plants [3]. Waterlogging stressors, commonly known as flooding stress, affect the species distribution and growth of many different communities of plants [5]. Heat and cold stresses negatively affect plant development. Among these, heavy metal toxicity is of more serious concern since it affects plant growth and development resulting in food safety and food security. Heavy metals refer to metals and metalloids that have higher densities, typically more than 5 g/cm3 [6]. These metals, when taken and absorbed by the plant root, cause many problems for plants, such as disturbed redox homeostasis, imbalanced electrolytes, electron transport chains, etc. [7]. Global climatic conditions are continuously changing, posing a serious threat to plant and human health. Therefore, it is necessary to compete with the changing climatic conditions in order to overcome environmental challenges and safeguard food security as well as food safety [8][9][10]. Plant growth and development are generally dependent on metabolic pathways, including REDOX processes, which allow them to synthesize their food in the form of ATPs [11]. However, when heavy metals (copper, arsenic, zinc, cobalt, chromium, manganese, etc.) are present in the soil, they ultimately disrupt the plant metabolism which results in an inhibition of plant growth [12][13][14][15][16][17]. These heavy metals are highly insoluble and remain in the soil for long periods. In the process of water uptake, heavy metals enter via root cells into the xylem, hindering plant metabolic pathways, and ultimately leading to plant cell death [18]. Phytohormones are known to regulate plant growth and development and induce stress tolerance against the aforementioned stresses. Among them, jasmonic acid (JA) is one of the vital hormones that has a role in plant signaling pathways which can induce biochemical and physiological modifications and helps plants mitigate the lethal effects of various abiotic stresses [19]. Many studies have been conducted on the specific role of exogenous and endogenous JA against abiotic stresses in plants. Nevertheless, understanding the complex processes that regulate the synthesis and function of JA in plants is a highly challenging process characterized by numerous intricate stages and molecular pathways. The activation of diverse gene actions intricately regulates the generation of JA.
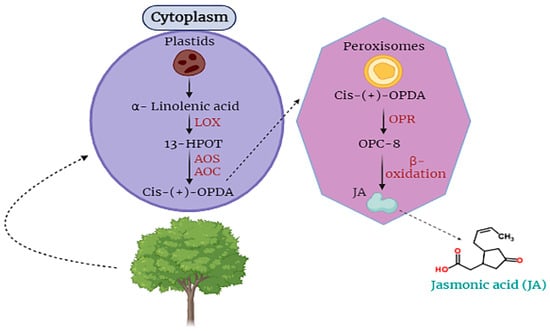 Moreover, jasmonic acid (JA) is synthesized within cellular compartments through enzymatic catalysis mediated by enzymes such as lipoxygenase and allene oxide cyclase, which govern the regulation of octadecanoid metabolic pathways. The process of signaling and releasing JA takes place in the plastids where α-linolenic acid is oxygenized into 13S-hydroperoxyoctadecatrienoic acid by the catalysis of LOX, then 13-HPOT is converted into the form of cis-(+)-12-oxophytodienoic acid by the activity of AOC and AOS. Then, OPDA is translocated to peroxisomes where OPDA is completely reduced by OPR (Figure 1).
Moreover, jasmonic acid (JA) is synthesized within cellular compartments through enzymatic catalysis mediated by enzymes such as lipoxygenase and allene oxide cyclase, which govern the regulation of octadecanoid metabolic pathways. The process of signaling and releasing JA takes place in the plastids where α-linolenic acid is oxygenized into 13S-hydroperoxyoctadecatrienoic acid by the catalysis of LOX, then 13-HPOT is converted into the form of cis-(+)-12-oxophytodienoic acid by the activity of AOC and AOS. Then, OPDA is translocated to peroxisomes where OPDA is completely reduced by OPR (Figure 1).
2. Plant Hormones Play Significant Roles in Stress Alleviation
Phytohormones, also known as plant hormones, are intrinsic chemical compounds that govern various physiological and biochemical plant processes when present in minimal concentrations. There are nine different classes of phytohormones reported [20][21][22]. According to their structural and chemical diversity, they are classified into auxin, cytokinins (CKs), ethylene, gibberellins (GAs), abscisic acid (ABA), salicylic acid (SA), brassinosteroids (BRs), strigolactones (SLs), and jasmonic acid [23]. Phytohormones play a very crucial role in regulating normal plant growth and maintaining the homeostasis of plant cells against abiotic stress. They are bio-stimulators and signaling molecules that induce the exocytosis process of cells under adverse conditions [24]. In addition to increasing plants’ resistance against abiotic stresses, like salinity and drought, phytohormones also perform vital regulatory roles against hazardous compounds [25][26]. Phytohormones protect plants against cellular-oxidative stress by increasing photosynthesis, and total chlorophyll content, as well as plant development and growth while reducing the production of reactive oxygen species [27]. Ionic toxicity is a direct consequence arising from the excessive accumulation of Na+ in plants subjected to saline conditions. The physiological integrity of plant cells relies on the particular maintenance of intracellular ionic equilibrium, a balance that can be disrupted by such conditions [28]. To uphold ionic homeostasis, jasmonic Acids (JAs) play a pivotal role. Notably, the introduction of JA to maize seedlings experiencing Na2CO3-induced stress has been observed to mitigate Na+/K+ ratios in both roots and leaves. This mitigation, in turn, alleviates ionic toxicity and reduces the harmful effects associated with alkaline stress [29]. Exogenous methyl jasmonate (MeJA) emerges as a crucial factor in preserving ion homeostasis in seedlings treated with NaCl, evidenced by its capacity to reduce Na+ accumulation and modulate the distribution of K+ between roots and shoots. This modulation results in a substantial decrease in the shoot Na+/K+ ratio. The accumulation of K+ in the shoots of salt-stressed plants enables them to sustain vital processes such as photosynthesis, metabolism, and osmotic pressure, concurrently mitigating ion toxicity [30]. These hormones induce different responses according to their mode of action.3. Jasmonic Acid as a Plant Biostimulant
Jasmonic acid is an endogenous biomolecule in plants that serves as a regulator of plant growth, initially characterized as a hormone associated with stress responses in higher plant species. They were first discovered in plants in 1962 and were identified to play a role in plant biological systems such as stress tolerance, movement, and reproductive growth of the plant cells [31][32][33]. These are carboxylic acids and hormonal lipids in nature, belonging to the class of oxylipins. Jasmonic acid and its derivatives are overall called jasmonates (JAs). Some of these derivatives include cis-jasmone, methyl jasmonate, jasmonate-amino acid, lactones of 12-hydroxy-JA-IIe, 12-O-glucosyl-JA-IIe, and jasmonic Isoleucine, etc. [34][35][36][37]. They are derived from the cyclopentanones compounds which link to the family of oxylipins. Therefore, these molecules are categorized as a family of lipids that play a vital role in cell signaling against abiotic stresses such as drought, salinity, heavy metals, etc. The concentration of JA varies in plant tissue. For example, in flowers and reproductive parts, it is very high, whereas its concentration is low in mature roots and leaves [11][38]. The role of JA is very crucial since it has multiple functions in plant cells like cell division, reproductive parts growth, fruit ripening, phosphorous and nitrogen uptake, electron transport chain, stomatal opening, and glucose transport [39]. In conclusion, JA has the ability to induce tolerance against heavy metals like lead (Pb), nickel, cadmium, and copper in different plant species, underscoring its potential for use in phytoremediation and stress management strategies. However, the molecular mechanisms through which JA exerts its effects on plant cells are poorly understood. A more in-depth discussion of the signaling pathways and target genes involved in JA-mediated stress responses would enhance the scientific understanding of this hormone.4. Biosynthesis of Jasmonic Acid
Jasmonic acid is an essential hormone that is found in plant cells. It is involved in the responding capability of plant cells against stress [18][40][41]. For the first time, JA was obtained from the Jasminum grandiflorum in the form of methyl ester JA. JA is categorized as a cyclopentane fatty acid. It is produced from α-linolenic acid, an important part of different membranes of plant cells (Figure 1) [42][43][44].
Figure 1.
Biosynthesis and translocation of jasmonic acid (JA) in plant cells.
5. Signaling Transduction of Jasmonic Acid
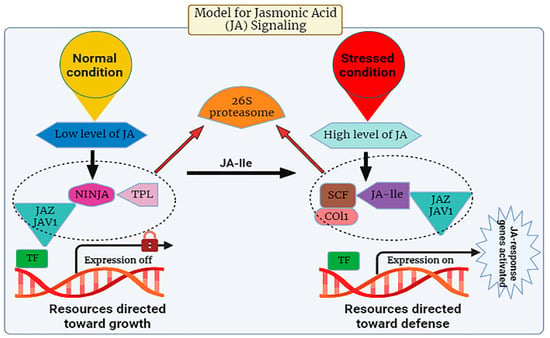
Many investigations have been conducted to unravel the signaling pathway of JA in plants. It is evident from studies that in response to abiotic stress, there is a prompt induction of genes associated with the synthesis of JA, leading to the activation of the plant’s defense mechanism through the regulated accumulation of JA within plant cells [45][46][47][48]. There are specific stress-responsive genes that switch on upon exposure to stress. The pathway of JA release is composed of CORONATINE INSENSITIVE 1(COI1), a receptor, JASMONATE ZIM-DOMAIN PROTEIN (JAZ) repressors, and the JA transcription factor known as MYC2. During its expression, the JA-Ile intermingles with the JA receptor (COI1). This COI1 is the F-box protein that works in the E3-ubiquitin ligase-mediated proteolysis of the JAZs. For the signaling pathway, MYC2 is known as a vital transcription factor involved in this process (Figure 2). The suppression effect of jasmonic acid genes was observed in Arabidopsis when the phenotype of the mutants of jasmonate insensitive 1 (jin1) was analyzed in 1996 [49].
Figure 2.
The expression levels of jasmonic acid under normal and stressed conditions.
6. Role of JA during Plant Defense Responses
The JA signaling pathway involves critical components, such as COI1, JAZ repressors, MYC2 as a vital transcription factor and the role of JA-Ile in activating this pathway. However, more detailed information on how COI1, JAZs, MYC2, and other components interact would enhance this clarity.
In the dynamic and constantly changing natural environment, plants may experience a multitude of biotic factors, including a wide array of pathogens, encompassing biotrophic, hemi-biotrophic, and necrotrophic pathogenic organisms, which are detrimental to the optimal development of plants
[50]
. Plant resistance responses to these stresses, especially to insects, are controlled by molecular signals, among which the most significant signaling molecule is JA. Certainly, lipid-derived jasmonates (JAs) play a pivotal role in various aspects of plant development and serve as integral components in the plant’s defense mechanisms against diverse pathogens, specifically necrotrophic fungal pathogens such as
Alternaria brassicicola
,
Botrytis cinerea
,
Plectosphaerella cucumerina
, and
Pythium
spp. These pathogens pose substantial threats to plant viability and growth
. Pathogen attack stimulates the biosynthesis of jasmonoyl-L-isoleucine (JA-Ile), which subsequently engages with the COI1-JAZ receptor, instigating the degradation of JAZ repressor proteins and the commencement of transcriptional processes associated with the activation of plant defense mechanisms (
Figure 3
).
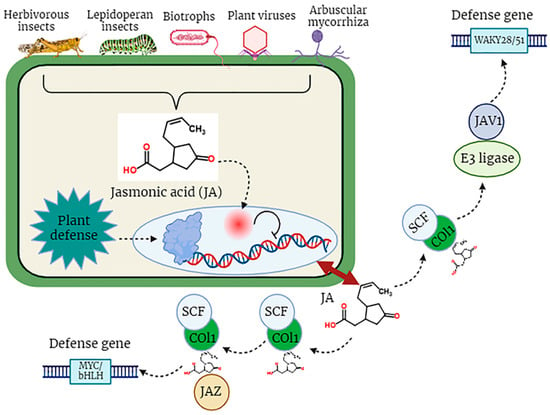

Figure 3. The defensive role of jasmonic acid (JA) in plants against biotic stress factors.
The JA signaling pathway comprises two distinct phases: repression, which occurs under normal physiological conditions, and activation, which is triggered in response to stress conditions. During normal conditions, the cytoplasm typically maintains a low concentration of JA-Ile, thereby causing the genes associated with JA to remain in an inactive state
[53]
. Gene promoters exhibit an affinity for various transcription factors (TFs), which are subjected to repression by various transcriptional repressors known as JAZ proteins, a group characterized by the presence of the jasmonate ZIM domain
[54]
. JAZ proteins initiate the formation of an active, closed complex referred to as the JAZ–NINJA–TPL complex by recruiting the protein known as topless (TPL) and the adaptor protein specific to JAZ (NINJA). This complex avoids the start of jasmonate-responsive genes
[55]
. During stress, the phytohormone jasmonic Acid-Isoleucine (JA-Ile) exhibits an increased accumulation within the cellular cytosol. Subsequently, these JA-Ile molecules are transported across cellular membranes to access the nucleus, facilitated by the catalytic activity of jasmonic Acid Transfer Proteins, specifically AtJAT1/AtABCG16. This process marks the initiation of the JA signaling pathway
[53]
. Within the cellular nucleus, the SCF complex, composed of kinetochore protein 1 (SKP1), cullin 1 (CUL1), and an F-box protein, serves as an E3 ubiquitin ligase that plays a pivotal role in facilitating JA responses. The initial step involves the translocation of JA-Isoleucine (JA-Ile) into the nucleus, where it is recognized by the F-box protein COI1, an integral component of the SCF complex.
7. Role of Jasmonic Acid under Abiotic Stresses in Plants
The specific roles of JA under abiotic stresses of plants have been investigated broadly. JA has the capacity to enhance the tolerance of plants against a wide spectrum of abiotic stress factors, including, but not limited to, drought, salinity, high temperature, cold, and stress induced by heavy metals
[50]
. JA and its derivatives play a crucial role in a plant’s defense against both biotic and abiotic stresses. The functions performed by JAs in protection growth and mobilizing plant defense responses constitute a direct path for stress reduction. In response to abiotic stressors, JAs primarily enhance tolerance by activating the plant defense mechanisms, mostly characterized by the induction of antioxidant enzymes and other protective compounds (
Figure 4
)
[53]
. The use of jasmonates as a foliar application could help improve the plants’ capability to mitigate the effects of abiotic stresses in different plants. There is an enhanced negative impact of heavy metals when they are contaminated in the growth medium of soil for plants
[12]
. The plant showed decreased physiological growth which leads to stunned height and fruit falling at an early stage
[56]
. For example, applying the chromium (150 and 300 µM) in Choy sum (
Brassica parachinensis
) caused a deep reduction in the root and shoot length, biomass, and plant growth. It also showed leaf necrosis and death of plant cells. Moreover, it also altered the composition of the antioxidant enzymes like DHAR, HAR, GST, SOD, APX, and many others
.
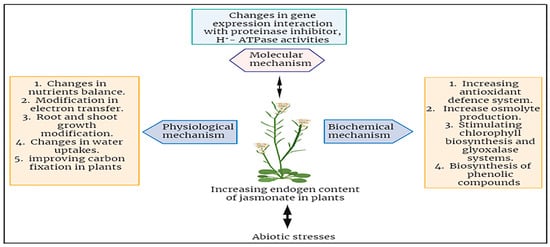

Figure 4. Summary of jasmonates mechanisms in enhancing abiotic stress tolerance in plants.
In conclusion, it highlights the beneficial effects of jasmonic acid (JA) in enhancing plant tolerance to various abiotic stresses, encompassing drought, salinity, heat, cold, and heavy metal stress. However, there is a need for further experiments to fully understand JA’s involvement in mitigating abiotic.
8. Conclusions
Plants have evolved special phytohormones, such as jasmonic acid (JA), that confer tolerance primarily by activating plant defense mechanisms, especially antioxidant enzymes. Further, the interactive mode of JA with these enzymes protects plants against the toxic effects caused by abiotic as well as biotic factors. Exogenous JA is regarded as a bio-stimulant that induces the expression of JA-related genes under drought, salinity, flooding, and heavy metal stress environments. Genetic modification is also a possible approach to a substantial upsurge in the production of JA leading to an enhanced resistance mechanism in plants. Recent advances in transcriptomics, genomics, and proteomics have unraveled the interaction of gene and protein networks. These techniques have also unfolded the synergistic crosstalk of JA with other phytohormones such as ABA, ET, and SA, etc. However, the behavior of phytohormones and their signaling networks are unpredictable and respond differently to different stress factors.
References
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621.
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021.
- Afridi, M.S.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; Sultan, T.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577.
- Salam, A.; Ali, A.; Afridi, M.S.; Ali, S.; Ullah, Z. Agrobiodiversity: Effect of Drought Stress on the Eco-physiology and Morphology of Wheat. In Biodiversity, Conservation and Sustainability in Asia: Volume 2: Prospects and Challenges in South and Middle Asia; Öztürk, M., Khan, S.M., Altay, V., Efe, R., Egamberdieva, D., Khassanov, F.O., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 597–618.
- Voesenek, L.; Rijnders, J.; Peeters, A.; Van de Steeg, H.; De Kroon, H. Plant hormones regulate fast shoot elongation under water: From genes to communities. Ecology 2004, 85, 16–27.
- Hawkes, S.J. What is a “heavy metal”? J. Chem. Educ. 1997, 74, 1374.
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322.
- Wang, F.; Yu, G.; Liu, P. Transporter-mediated subcellular distribution in the metabolism and signaling of jasmonates. Front. Plant Sci. 2019, 10, 390.
- Oshita, T.; Sim, J.; Anee, T.I.; Kiyono, H.; Nozu, C.; Suzuki, N. Attenuation of negative effects caused by a combination of heat and cadmium stress in Arabidopsis thaliana deficient in jasmonic acid synthesis. J. Plant Physiol. 2023, 281, 153915.
- Munir, R.; Jan, M.; Muhammad, S.; Afzal, M.; Jan, N.; Yasin, M.U.; Munir, I.; Iqbal, A.; Yang, S.; Zhou, W. Detrimental effects of Cd and temperature on rice and functions of microbial community in paddy soils. Environ. Pollut. 2023, 324, 121371.
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A.J.P. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825.
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S.; et al. Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L. (Merr.) roots. Environ. Pollut. 2023, 317, 120637.
- Azhar, W.; Khan, A.R.; Salam, A.; Ulhassan, Z.; Qi, J.; Shah, G.; Liu, Y.; Chunyan, Y.; Yang, S.; Gan, Y. Ethylene accelerates copper oxide nanoparticle-induced toxicity at physiological, biochemical, and ultrastructural levels in rice seedlings. Environ. Sci. Pollut. Res. 2023, 30, 26137–26149.
- Khan, A.R.; Azhar, W.; Wu, J.; Ulhassan, Z.; Salam, A.; Zaidi, S.H.R.; Yang, S.; Song, G.; Gan, Y. Ethylene participates in zinc oxide nanoparticles induced biochemical, molecular and ultrastructural changes in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 226, 112844.
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic acid underpins silicon in ameliorating chromium toxicity in rice by modulating antioxidant defense, ion homeostasis and cellular ultrastructure. Plant Physiol. Biochem. 2021, 166, 1001–1013.
- Zeeshan, M.; Hu, Y.X.; Iqbal, A.; Salam, A.; Liu, Y.X.; Muhammad, I.; Ahmad, S.; Khan, A.H.; Hale, B.; Wu, H.Y.; et al. Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol. Environ. Saf. 2021, 225, 112738.
- Salam, A.; Rehman, M.; Qi, J.; Khan, A.R.; Yang, S.; Zeeshan, M.; Ulhassan, Z.; Afridi, M.S.; Yang, C.; Chen, N. Cobalt stress induces photosynthetic and ultrastructural distortion by disrupting cellular redox homeostasis in maize. Environ. Exp. Bot. 2023, 2023, 105562.
- Piotrowska, A.; Bajguz, A.; Godlewska-Żyłkiewicz, B.; Czerpak, R.; Kamińska, M.J.E.; Botany, E. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ. Exp. Bot. 2009, 66, 507–513.
- Zuo, Z.-F.; Lee, H.-Y.; Kang, H.-G. Basic Helix-Loop-Helix Transcription Factors: Regulators for Plant Growth Development and Abiotic Stress Responses. Int. J. Mol. Sci. 2023, 24, 1419.
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468.
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098.
- Ahmad, M.A.; Gupta, M.J.E.S.; Research, P. Exposure of Brassica juncea (L) to arsenic species in hydroponic medium: Comparative analysis in accumulation and biochemical and transcriptional alterations. Environ. Sci. Pollut. Res. 2013, 20, 8141–8150.
- Chen, J.; Yan, Z.; Li, X.J.E.; Safety, E. Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 104, 349–356.
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39.
- Sezer, I.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376.
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283.
- Kul, R.; Esringü, A.; Dadasoglu, E.; Sahin, Ü.; Turan, M.; Örs, S.; Ekinci, M.; Agar, G.; Yildirim, E. Melatonin: Role in increasing plant tolerance in abiotic stress conditions. Abiotic Biot. Stress Plants 2019, 1, 19.
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349.
- Mir, M.A.; John, R.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci. Rep. 2018, 8, 2831.
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445.
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125.
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39.
- Shekhawat, K.; Fröhlich, K.; García-Ramírez, G.X.; Trapp, M.A.; Hirt, H. Ethylene: A Master Regulator of Plant–Microbe Interactions under Abiotic Stresses. Cells 2023, 12, 31.
- Alirzayeva, E.; Neumann, G.; Horst, W.; Allahverdiyeva, Y.; Specht, A.; Alizade, V. Multiple mechanisms of heavy metal tolerance are differentially expressed in ecotypes of Artemisia fragrans. Environ. Pollut. 2017, 220, 1024–1035.
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641.
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381.
- Koda, Y. The role of jasmonic acid and related compounds in the regulation of plant development. Int. Rev. Cytol. 1992, 135, 155–199.
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L.; Research, P. Phytoremediation of toxic metals present in soil and water environment: A critical review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860.
- Pandey, N. Role of plant nutrients in plant growth and physiology. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 51–93.
- Xia, Y.; Liu, J.; Wang, Y.; Zhang, X.; Shen, Z.; Hu, Z.J.E.; Botany, E. Ectopic expression of Vicia sativa Caffeoyl-CoA O-methyltransferase (VsCCoAOMT) increases the uptake and tolerance of cadmium in Arabidopsis. Environ. Exp. Bot. 2018, 145, 47–53.
- Shri, M.; Singh, P.K.; Kidwai, M.; Gautam, N.; Dubey, S.; Verma, G.; Chakrabarty, D. Recent advances in arsenic metabolism in plants: Current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics 2019, 11, 519–532.
- Barcelo, J.; Poschenrieder, C.J.E.; Botany, E. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92.
- Guo, T.R.; Zhang, G.P.; Zhang, Y.H. Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf. B Biointerfaces 2007, 57, 182–188.
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis–structure, function, regulation. Phytochemistry 2009, 70, 1532–1538.
- Fan, X.; Mattheis, J.P.; Fellman, J.K. Responses of apples to postharvest jasmonate. J. Am. Soc. Hortic. Sci. 1998, 123, 421–425.
- Zhao, Q.; Sun, Q.; Dong, P.; Ma, C.; Sun, H.; Liu, C. Jasmonic acid alleviates boron toxicity in Puccinellia tenuiflora, a promising species for boron phytoremediation. Plant Soil 2019, 445, 397–407.
- Cohen, S.; Flescher, E. Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry 2009, 70, 1600–1609.
- Hu, Y.; Sun, H.; Han, Z.; Wang, S.; Wang, T.; Li, Q.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X. ERF4 affects fruit ripening by acting as a JAZ interactor between ethylene and jasmonic acid hormone signaling pathways. Hortic. Plant J. 2022, 8, 689–699.
- Berger, S.; Bell, E.; Mullet, J.E. Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol. 1996, 111, 525–531.
- VanWallendael, A.; Soltani, A.; Emery, N.C.; Peixoto, M.M.; Olsen, J.; Lowry, D.B. A molecular view of plant local adaptation: Incorporating stress-response networks. Annu. Rev. Plant Biol. 2019, 70, 559–583.
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385.
- Yan, C.; Xie, D. Jasmonate in plant defence: Sentinel or double agent? Plant Biotechnol. J. 2015, 13, 1233–1240.
- Ali, M.S.; Baek, K.-H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621.
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449.
- Ke, J.; Ma, H.; Gu, X.; Thelen, A.; Brunzelle, J.S.; Li, J.; Xu, H.E.; Melcher, K. Structural basis for recognition of diverse transcriptional repressors by the TOPLESS family of corepressors. Sci. Adv. 2015, 1, e1500107.
- Avalbaev, A.; Allagulova, C.; Maslennikova, D.; Fedorova, K.; Shakirova, F. Methyl jasmonate and cytokinin mitigate the salinity-induced oxidative injury in wheat seedlings. J. Plant Growth Regul. 2021, 40, 1741–1752.
- Kurowska, M.M.; Daszkowska-Golec, A.; Gajecka, M.; Kościelniak, P.; Bierza, W.; Szarejko, I. Methyl jasmonate affects photosynthesis efficiency, expression of HvTIP genes and nitrogen homeostasis in barley. Int. J. Mol. Sci. 2020, 21, 4335.
- Napoleão, T.A.; Soares, G.; Vital, C.E.; Bastos, C.; Castro, R.; Loureiro, M.E.; Giordano, A. Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 2017, 263, 46–54.
More
