1. Recycled Polystyrene
Polystyrene is a polymer often employed in the form of foamed polystyrene known as styrofoam, composed of 2% polymer and 98% air
[1][90]. Styrofoam is primarily used in disposable utensils production, building insulation, making food packing containers, packing delicate items, etc
[2][3][91,92]. Annual production of approximately 14.7 and 6.6 million metric tons (MMT) in 2016 was reported for polystyrene and foamed polystyrene, respectively
[4][93]. The passage of time might have enhanced its utilization and subsequent annual waste generation. Unfortunately, post-consumer waste is disposed of in the municipal landfill, creating a substantial waste quantity and posing an environmental burden. Additionally, most plastics take a considerable length of time to decompose, often lasting several hundred years
[5][94].
Ghaly et al. carried out a comparative study using two polystyrene wastes (i.e., transparent and white colors derived from the used yogurt packaging cups) and DMF as a solvent to fabricate polymeric membranes while using membranes fabricated via commercial polystyrene pellets as a control. The utilization of both polymer materials, regardless of their transparency or white color, does not significantly deviate from commercial polystyrene membranes when fabricated using the nonsolvent-induced phase separation (NIPS) technique. The results demonstrated that the polystyrene membranes synthesized from both sources had comparable overall characteristics and thus could be effectively used for the production of flat-sheet membranes
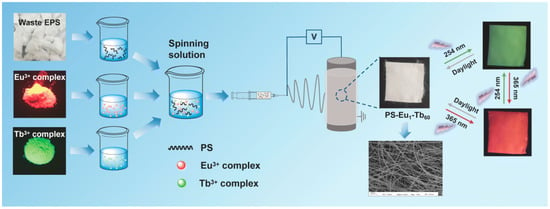
[6][42]. Fan et al. reported UV-excited dual-mode multicolor luminescent electrospun fiber membrane fabrication using waste-expanded polystyrene (2.5 g dissolved in 2.5 mL DMF) by co-doping with a Europium (Eu
3+) complex and terbium (Tb
3+) complex via electrospinning (
Figure 15)
[7][88]. Under UV light excitation, the luminescence analysis results indicated that all the as-prepared fiber membranes with varying mass ratios of the two complexes could emit Eu
3+ ions and Tb
3+ ions characteristically. As illustrated in
Figure 15, the fiber membranes exhibited strong visible luminescence of different colors when exposed to 254 nm and 365 nm UV light. Ramos-Olmos et al. manufactured membranes via phase inversion using foamed polystyrene, N-methyl-2-pyrrolidone (NMP), and PEG and dioctyl phthalate as polymer, solvent, and additives, respectively
[8][95]. The presence of additives altered the modified membranes’ surface properties which subsequently resulted in greater water permeability compared to the normal polystyrene used as a control.
Figure 15. Waste expanded-polystyrene-derived membrane fabrication [7]. Reprinted with copyright permission. Waste expanded-polystyrene-derived membrane fabrication [88]. Reprinted with copyright permission.
The use of recycled polystyrene with functionalized carbon nanotubes and DMF (solvent) for fabricating UF membranes to treat river water was also reported by Adamczak et al.
[9][96]. The results obtained indicated that pristine polystyrene membranes possessed comparable surface properties to membranes composed of other polymers and could be utilized indiscriminately with them. The outcomes achieved via surface water purification exhibited similarities to those documented in other studies employing commercial membranes. The membranes have shown a significant capacity to extract phenolic chemicals, suggesting a strong potential for effectively eliminating these pollutants. Ke et al. fabricated hydrophobic electrospun polystyrene nanofibrous membranes (fabricated using four polystyrene solutions of varying concentrations prepared in MDF) for desalination utilizing the DCMD configuration
[10][97]. The developed membrane effectively desalinated saltwater, showing over 99.99% rejection. The water flow through the membrane dropped as the membrane thickness increased and the pore size decreased under the applied conditions for all types of feed solutions. Huan et al. created electrospun polystyrene fibers by dissolving polystyrene beads in a combination of DMF and tetrahydrofuran
[11][98]. It was observed that augmenting the ratio of tetrahydrofuran to DMF led to the production of more robust and smoother fibers without any irregularities. This phenomenon was attributed to the creation of a polymer solution with higher viscosity as a result of the increased proportion of these solvents. The authors further examined the impact of the electrospinning voltage on the synthesis process and observed enhancement in generated mat hydrophilicity when changed from 12 to 20 kV. The microscopic analysis of the manufactured fibers revealed the presence of intertwined fibers arranged in a strand-like configuration, which was identified as the underlying cause for the observed high tensile strength of 1.5 MPa. Although the efficacy of the fiber mats for water treatment was not examined by the authors, their work yielded significant findings that can be highly advantageous in the development of recycled polystyrene membranes.
2. Recycled Polyethylene Terephthalate
Polyethylene terephthalate (PET) is one of the most widely used polymers. PET has been used in various applications due to its chemical stability in acids and organic solvents, remarkable mechanical properties, high transparency, and good gas-barrier resistance
[12][99]. The PET bottles can potentially be used for fabricating the membranes
[13][100]. Such repurposing of this waste not only helps to decrease the waste of plastic bottles but also reduces polymer consumption, associated greenhouse gas emissions, and membrane production costs
[14][101]. The use of PET to fabricate a non-woven support layer of electrospun fiber
[15][102], ion tracked-etched porous membrane for making asymmetric multifunctional heterogeneous structures
[16][103], mechanically stable braid-reinforced fiber membranes
[17][104], and several membrane types are reported by the authors
[18][19][20][105,106,107]. The use of unmodified PET for fabricating the membranes demonstrated poor mechanical properties. However, the addition of PEG with higher molecular weights resulted in higher permeation flux and a stronger structure
[21][108]. Using recycled PET as polymer and phenol, m-cresol, or dimethyl sulfoxide as a solvent, Kusumocahyo et al. fabricated UF membranes via phase inversion. Their results showed that lowering the polarity of the nonsolvent increased water permeation. The constructed membrane also demonstrated better flux when concentrations and the molecular weight of the additives in the casting solution increased
[22][109].
Fabrication of the UF membrane was demonstrated in another study using recycled PET bottles as polymer, phenol as a solvent, PEG as an additive, and water/ethanol as a coagulation nonsolvent
[23][110]. An increase in porosity and hydrophilicity was observed when the PEG concentration changed, although the pore size of the membrane was reduced. These changes brought about an increase in pure water flux and BSA solution rejection. Zander et al. fabricated an electrospun nanofiber MF membrane using recycled PET (dissolved in Hexafluoro-isopropanol and tri-butylammonium chloride) for latex beads separation (ranging from 30 to 2000 nm)
[24][111]. The prepared membrane demonstrated 99% bead separation with only gravity filtration. Xu et al. obtained a hydrophobic (contact angle ≥ 130°) electrospun nanofiber membrane for MD when using recycled Coca-Cola bottles (dissolved in trifluoroacetic acid) and performed fluorination (
Figure 26a). As shown in
Figure 26b, the prepared membrane demonstrated higher flux and rejection (11–23 LMH and higher rejection, almost 100%) when compared to pristine (10 LMH and 95%, respectively)
[25][112]. Excellent resistance toward acidic and oxidative agents in the presence of solvents such as DMF at a high temperature (100 °C) was observed by Pulido et al. when performing PEG/water and PEG/DMF filtration using recycled PET-derived UF membranes. This reflects the potency of recycled PET-derived membranes in applications where high temperature and corrosive materials are present
[26][113].
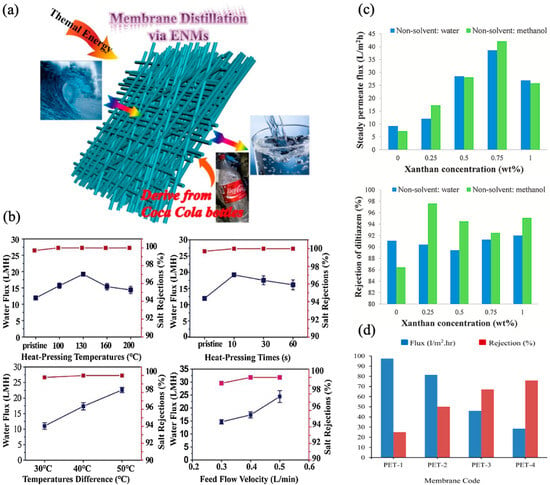
Figure 26. Recycled PET-derived membrane: (
a) schematic illustration; (
b) water flux (blue line) and rejection (pink line) at different heat pressing temperatures (10 s duration), time (at 130 °C, ΔT = 40 °C, velocity = 0.4 L min
−1), temperature difference (feed velocity = 0.4 L min
−1), and feed flow velocity (ΔT = 40 °C)
[25][112]; (
c) flux and diltiazem rejection of PAT/XA membrane
[27][114]; (
d) flux and humic acid rejection of recycled PET/polyvinylpyrrolidone membranes
[14][101]. Reprinted with copyright permission.
Using PET/XA (Xanthan gum) and trifluoroacetic acid with two nonsolvent solutions, methanol and water, Kiani et al. fabricated an NF membrane to demonstrate diltiazem removal from an aqueous solution. The addition of XA (0.25–0.75 wt%) into the casting solution enhanced membrane porosity, thickness, and hydrophilicity, although further enhancement in XA (1 wt%) affected the membrane properties adversely
[27][114]. Adding XA made the membrane structurally weaker, as indicated by tensile and elongation tests. Membranes prepared with water and methanol with XA of 0.75 wt% had the highest steady flux of ≈38 and 42 LMH, respectively. Nevertheless, the maximum rejection for diltiazem (i.e., 98% and 92%) was seen when using a PET membrane with 0.25 wt% XA dissolved in methanol and 1 wt% XA dissolved in water, respectively (
Figure 26c). Utilizing the thermally induced phase separation (TIPS) method, Arahman et al. fabricated a UF membrane using recycled PET. The addition of polyvinylpyrrolidone (5 wt%) produced a sponge-like structure in the membrane sublayer, while casting without evaporation resulted in a more porous membrane. Although membranes without PVP had higher flux (97 LHM), better HA rejection (76%) was obtained when adding the 5 wt% PVP (
Figure 26d). This study highlights the potency of recycled PET membranes for surface water treatment; however, further refining of the synthesis and modification processes is still required to produce high-performing membranes
[14][101].
3. Recycled Polyvinyl Chloride
Polyvinyl chloride (PVC) is another widely used material known for its extended durability and favorable characteristics in terms of mechanical, electrical, chemical, and thermal resistance. PVC finds use in several sectors, such as building and construction, automotive, pipeline, cable industries, and the manufacturing of numerous home items. The PVC material possesses exceptional strength, durability, lightness, and versatility, rendering it an ideal choice for a multitude of applications. Several studies have utilized neat PVC for fabricating the membranes owing to its extraordinary characteristics
[28][29][30][31][115,116,117,118]. However, less attention has been paid to making the membranes using waste PVC. The feasibility of utilizing waste PVC as a polymer source for fabricating the UF membrane was established. Prior to the phase inversion process being employed to produce the membrane, PVC waste acquired from post-consumer disposal was dissolved in DMF. Subsequently, different concentrations of cellulose acetate (CA) were employed to minimize the hydrophobic characteristics of PVC. The PVC UF membrane created from waste plastic achieved a BSA rejection rate of 91%, which is comparable to other PVC membranes made from commercially available precursors. The pure water flux and flux recovery ratio showed an upward trend as the CA concentration rose from 0 to 5 wt%. The pure water flux rose from 36 L m
−2 h
−1 to 85 L m
−2 h
−1, while the flux recovery ratio increased from 56% to 78%. These results suggest that neat PVC with high hydrophobicity negatively affected water permeability and anti-fouling capabilities
[32][119].
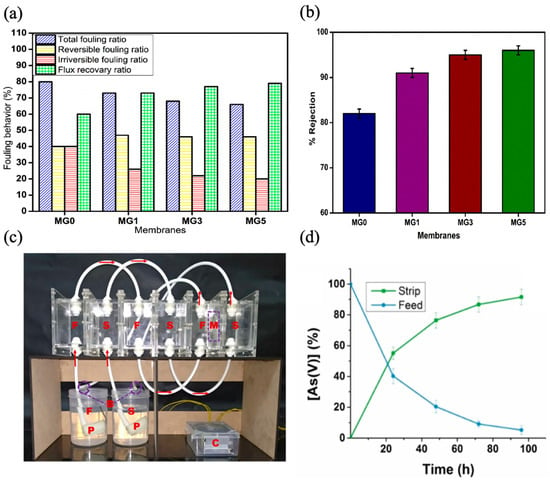
In a separate study, Aji et al. investigated the use of waste PVC as a membrane substrate and the enhancement of its hydrophilicity via the incorporation of gum Arabic, a sustainable biopolymer, for the removal of natural organic matter in water. The researchers employed the phase inversion method to prepare the membranes while adjusting the proportion of gum Arabic in a solution containing PVC and N-methyl pyrrolidone. The interactions between waste PVC and gum Arabic at various loadings (1–5% wt.%) were analyzed using a variety of analytical techniques. The GA-incorporated PVC membrane demonstrated a 25% increase in its hydrophilicity. The water permeation improved from 51 L m
−2 h
−1 to 98 L m
−2 h
−1, accompanied by a flux recovery ratio of around 80% and a rejection rate of roughly 96% (
Figure 37a,b). Additionally, the onset degradation temperature saw an increase of around 30 °C, while the mechanical strength was strengthened by approximately 1.2 MPa
[33][120]. Govindappa et al. demonstrated the integration of recycled PVC material as a base polymer and benzalkonium chloride (BAC) as an extractant to develop low-cost polymer inclusion membranes for extracting arsenic from water
[34][121]. The polymer inclusion membrane, composed of 50% PVC dissolved in tetrahydrofuran, 40% BAC, and 10% Dioctyl phthalate, had the best transport efficiency for arsenic among membranes with varying compositions. The membrane facilitated the arsenic transport from an aqueous solution (pH = 7) to a 2 M NaCl stripping solution and showed 57.8% efficiency during a 24 h period. The membrane demonstrated exceptional transport efficiency for various anions found in water at lower concentrations when replacing 10% recycled PVC with base polymer. Nevertheless, a decrease in efficiency was noted at higher concentrations. The membrane exhibited excellent resistance to biofouling. To ensure a continuous conveyance process, a prototype capable of operating without any intervention is developed (
Figure 37c); its efficiency was estimated to be approximately 91% over the course of 100 h (
Figure 37d). Following five cycles, the device maintained an overall efficacy of over 80%. The findings indicate that the stability and performance of the pure PVC membrane were not influenced by the incorporation of recycled PVC, in contrast to the standard polymer inclusion membrane that did not contain recycled PVC.
Figure 37. Recycled PVC membranes performance: (
a) flux recovery ratio; (
b) humic acid rejection (MG0, MG1, MG3, and MG5 refers to the membranes fabricated with different gum Arabic concentrations ranging from 0–5%, respectively)
[33][120]; (
c) prototype device used for experiments (where F = feed solution; S = strip solution; M = membrane; P = pump; C = controller; B = feedback of feed and strip solution; (
d) percent arsenic transport
[34][121]. Reprinted with copyright permission.
4. Recycled Tire Rubber
Tire production utilizes approximately 70% of the rubber produced globally. Considering that tires contain about 60 percent natural and synthetic rubber, an estimated 6 million tons of refuse tires are produced annually. The annual global quantity of waste tires disposed of is close to 800 million (10 million tons)
[35][122]. In an effort to achieve the objectives of resource recycling and environmental protection, developed nations have paid considerable attention to the ubiquitous use of discarded tires. Landfilling, incineration, pyrolysis, and devulcanization/reclaiming of waste rubber products have been suggested as potential solutions to the recycling and disposal issue associated with rubber waste. Since 1999, the disposal of tire waste in landfills has been prohibited by the European Commission. Out of the many methods considered, abandoning the material is the least desirable. Beyond that, the value added is negative, and this circumstance does not contribute to the product’s value. While it is possible to mitigate the hazardous pollution issue caused by refuse tires by utilizing them as a fuel source, this approach is not environmentally preferred. For instance, the incineration of waste rubber products generates secondary pollutants, including noxious odors and hazardous vapors, which contribute significantly to air pollution. Furthermore, the enormous quantity of CO
2 produced when waste rubber is incinerated contributes to global warming. Devulcanization and rubber waste reclamation are thus the most beneficial method for resolving the disposal issue. Devulcanization and reclamation serve the dual purpose of safeguarding habitats and conserving the nonrenewable petroleum resources that provide the basic materials for these processes
[36][37][123,124].
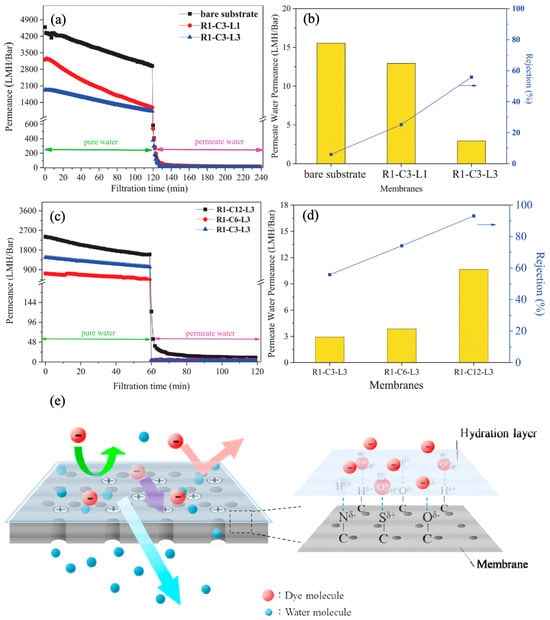
Using powder and devulcanized rubber derived from recycled tires, Lin et al. fabricated the composite hollow fiber NF membrane. The rubber selective layer was generated on the internal surface of the ceramic substrate subsequent to its dissolution in toluene. The layer served as a structural support, thereby enhancing the mechanical strength. The rubber casting solution, drawn into the porous substrate via the capillary effect, facilitated rubber adhesion to the substrate. The thermal and solubility properties of the rubber were predominantly influenced by the compositions of the waste rubber, as observed. The solubility of rubber powder was lower than that of devulcanized recycled rubber due to its crosslinked three-dimensional structure. An inverse relationship was observed between the number of internal coatings and permeability during filtration using MB dye solution; this relationship was attributed to the dense rubber selective layer (
Figure 48a,b). Conversely, an increase in rubber concentration from 6% to 12% resulted in a corresponding rise in the permeability of purified water (from 470.2 to 1596.1 L m
−2 h
−1) and the permeability of the dye solution (from 3.9 to 10.6 wt%) (
Figure 48c,d). This can be attributed to the augmented presence of surface hydrophilic groups, which contribute to the formation of the hydration layer (
Figure 48e). The hydration layer’s anti-fouling qualities facilitated permeance recovery, surpassing 80% after washing. The hydration layer functions as a barrier to reduce the adsorption of MB molecules onto the surface of the membrane. The non-vulcanized rubber-based NF membrane, containing 12% rubber concentration and coated internally three times, demonstrated a rejection rate of 93.1% for MB. Following three filtration cycles, the membrane’s permeability remained at a level of 8.3 L m
−2 h
−1 while achieving a 98.1% rejection rate for MB
[38][125]. In addition to the application of tire-derived polymeric membranes for treating the water, their utilization for gas separation is also reported elsewhere
[39][40][126,127].
Figure 48. Performance of recycled tire-derived membranes: (
a) permeance vs. filtration time; (
b) permeate permeance and dye rejection as a function of the internal coating; (
c) permeance vs. filtration time; (
d) permeate permeance and dye rejection as a function of precursor concentration; (
e) schematic representation of the separation mechanism by hydration layer
[38][125]. Reprinted with copyright permission. (R
1 refers to reclaimed rubber with devulcanization from a waste tire; C
3, C
6, and C
12 refer to %R
1 concentration used; and L
1 and L
3 refer to the number of internal coating cycles ranging from 1–3, respectively).
5. Recycled Keratin
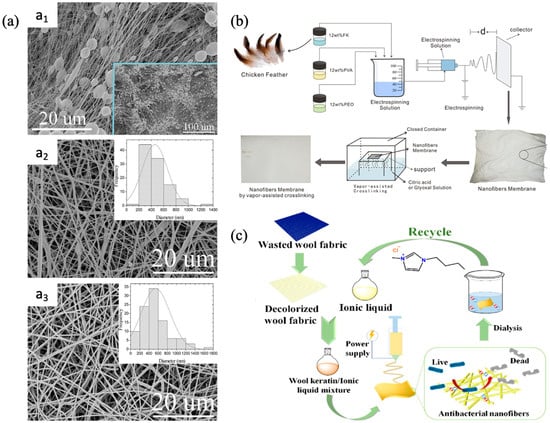
Chicken feathers, fleece, hair, hoof, and nails are the primary sources of keratin. The global accumulation of keratin refuse has steadily escalated into an unmanageable problem over time
[41][128]. Ma et al. fabricated the wool keratin (WK)/polyethylene oxide nanocomposite membrane using waste wool fabric
[42][129]. To enhance the spinnability of the wool keratin, the decolorized wool fabric was dissolved in formic acid containing poly(ethylene oxide) (PEO) followed by electrospinning at a rate of 0.5 mL h
−1, a syringe-collector distance of 15 cm, and a voltage of 14 kV, for fabricating the nanofiber membranes. The prepared membrane showed average diameter enhancement from 226 nm to 379 nm, and bead formation was inhibited when 10 wt% PEO was added (
Figure 59a). Ding et al. utilized keratin obtained from feathers, PEO, and PVA to fabricate membranes via electrospinning
[43][130]. The fabricated membranes were then crosslinked using citric acid and glyoxal vapor to produce NF membranes that are suitable for filtration applications requiring resistance to solvents (
Figure 59b). The mean diameter of the nanofibers exhibited an increase from about 223 nm in control to approximately 342 nm and 304 nm in the citric- and glyoxal-crosslinked membranes, respectively. This enlargement may be attributed to the diffusion of the crosslinked chains into the macromolecular chains, resulting in the formation of a crosslinking network. The process of crosslinking significantly increased the intermolecular connection of the composite membranes, resulting in higher mechanical and thermal stability.
Figure 59. Recycled keratin-derived membranes: (
a) morphology and diameter distribution of keratin/polyethylene oxide nanocomposite membrane ((
a1–
a3) refers to SEM images of 100/0 WK-PEO, 90/10 WK-PEO, and 70/30 WK-PEO nanofibrous membrane while of the inset figures depict diameter distribution of the respective membrane)
[42][129]; (
b) schematic illustration of electrospun membrane fabricated using chicken feather (d refers to the distance between the syringe needle and the collector)
[43][130]; (
c) stepwise illustration of wool fabric derived membranes
[37][124]. Reprinted with copyright permission.
Zong et al. reported the eco-fabrication of nanofibrous membranes with antibacterial and high moisture permeability using waste wool fabrics
[44][131]. The NF membrane fabrication involved the blending of wool fabric keratin (dissolved in 1-butyl-3-methylimidazolium chloride) with PAN (dissolved in DMF), followed by electrospinning of the mixture. The stepwise illustration of the fabrication mechanism is shown in
Figure 59c. The PAN/keratin nanofiber membrane showed significant antibacterial activity, with inhibition rates of 89.21% against
E. coli and 60.70% against
S. aureus, as determined by the bacterium colony count. In contrast, the PAN membrane without antibacterial capabilities did not demonstrate such effects. The moisture-management testing indicated that the PAN/keratin membrane exhibited enhanced wetting and moisture transport characteristics in comparison to the PAN membrane due to the inclusion of hydrophilic functional groups from keratin. In a separate study, the electrospinning of a polymer dope composed of a mixture of commercial PA and keratin extracted from waste goat hair was utilized to fabricate a nanofiber membrane for pigment removal
[45][132]. Sulfitolysis was employed to extract the keratin, and formic acid was utilized to dissolve the hydrolyzed keratin to produce the keratin solution. An interconnected pore structure nanofiber membrane featuring fine, irregularly oriented beads was subsequently produced via the electrospinning of the polymer material at a rate of 0.1 mL h
−1 and a distance of 22 cm using a 20 kV voltage. In contrast to the nanofiber composed solely of PA, the membrane composed of PA/keratin exhibited reduced crystallinity, increased porosity, and a smaller mean pore size. The reduced mechanical strength of the regenerated keratin was attributed to its rigid and stiff structure. When testing the filtration efficacy of the nanofiber membrane with PA/keratin to remove dark green anionic tannery dye, it consistently exhibited superior performance compared to the PA membrane. The highest dye rejection of 100% was achieved under acidic conditions using a 100 ppm dye solution. Nevertheless, this study did not specifically focus on the interaction and synergistic effects of PA and keratin that mutually enhanced performance. The use of keratin, extracted from the goat hair, for fabricating the NF membrane for dye removal is also reported elsewhere
[46][133].
6. Recycled Cellulose and Its Derivatives
The use of cellulose and its derivatives for fabricating the membranes has also been reported for environmental sustainability. Studies have reported the utilization of cellulose derived from oil palm empty fruit bunches, kapok fiber, non-dyed cotton bedsheets, cotton microdust waste released during the spinning process, waste paper, and waste cigarettes for fabricating the membranes. In order to remediate dye wastewater, Teow et al. developed a cellulose/PVDF membrane using oil palm empty fruit bunches containing 40–50% cellulose
[47][134]. The extracted cellulose was dissolved in solvents (N-Methyl-2-pyrrolidone (NMP) and N-N-dimethylacetamide (DMAc)), which was followed by lithium chloride addition. An increase in the cellulose-to-PVDF ratio in the dope led to a more rapid exchange of solvents and nonsolvents via the hydrophilic cellulose content. This was achieved by decreasing the viscosity of the dope and weakening the interaction between the hydrophobic PVDF and water. Consequently, the membrane developed wider pores. As a result of the increased solubility parameter, which accelerated the precipitation rate and encouraged the formation of a sponge-like structure, the membranes prepared with NMP solvent demonstrated greater porosity and larger pore size than those prepared with DMAc. The cellulose membrane, which was prepared using NMP solvent (cellulose-to-PVDF ratio of 96:4 and cellulose content of 3 wt%), demonstrated excellent properties such as a high permeate flux of 158.06 Lm
−2 h
−1 and an MB dye rejection rate of 34.9%.
Mohamed et al. successfully synthesized self-assembling cellulose nanocrystal membranes by extracting cellulose nanocrystals and utilizing kapok fiber as the cellulosic source (
Figure 610a)
[48][135]. By employing the water suspension casting evaporation method, the cellulose fibers acquired via acid hydrolysis and alkali extraction of kapok fiber were incorporated into a nanoporous membrane. The pretreatment resulted in a significant increase in the cellulose content, raising it from 59.6% in the raw kapok fiber to 92.8% in the hydrolyzed cellulose microfiber. This process also led to a reduction in the size of the fiber at the micro-scale level, resulting in the formation of a nanorod or nano-needle structure (
Figure 610a). By virtue of the function of surface hydroxyl groups in facilitating adsorption via hydrogen bonding or electrostatic interaction, the membrane demonstrated a highly encouraging removal efficiency of 85% when used to remove 5 ppm MB from an aqueous medium (
Figure 610b). Lopatine et al. demonstrated the fabrication of UF membranes (with a 150 µm thickness) using a non-dyed cotton bedsheet as cellulose (dissolved in 1–ethyl–3–methylimidazole acetate and dimethyl sulfoxide) and observed nearly 90% polyethylene glycol (35 kDa) retention
[49][136]. The obtained performance was comparable to the commercial and regenerated cellulose membranes. Vignesh et al. also utilized the cotton microdust released as waste during the spinning process in the textile industry to fabricate cellulose membranes
[50][137]. The rheology and tensile experiments demonstrated good mechanical characteristics. The authors ascribed this to the presence of a small lignin amount in the membrane. The prepared membrane also exhibited a high bactericidal effect against E. coli. This was attributed to the inclusion of Zn
2+ ions, which caused oxidative stress in the microbial cell. While the study did not specifically assess the separation performance, the physical properties and antibacterial test results make it a promising option for liquid separation due to its strong mechanical strength, resistance to bacterial fouling, and ability to attract water.
Figure 610. Membranes fabricated via cellulose and their derivates: (
a) morphology of kapok fiber derived membrane ((
a1–
a3) refers to raw kapok fiber, cellulose microfiber after NaOH treatment, and cellulose microfiber after NaClO
2 treatment, respectively. While (
a4–
a9) refer to their SEM morphologies at different resolutions); (
b) digital photo of methylene blue before and after adsorption; (
c) digital photos of the fabricated membrane showing
[48][135]; (
d) schematic illustration of CA (derived from cigarette) membrane fabrication and its performance evaluation
[51][138]. Reprinted with copyright permission.
Mohamed et al. fabricated the cellulose membrane by utilizing the non-printed area of recycled newspaper and dissolving it in NaOH (7 wt%)/urea (12%) aqueous solution
[52][139]. The resulting cellulose membrane exhibited a homogenous, symmetric, and dense structure, with mean porosity and pore size of 41.0% and 2.48 nm, respectively. However, due to its structural characteristics, the membrane exhibited low pure water permeability (0.35 m
−2 h
−1 bar
−1), indicating that more fine-tuning is necessary to increase the cellulose membrane’s suitability for filtering the liquid. In a subsequent study conducted by a similar author, the addition of N-doped TiO
2 nanorod to the newspaper-derived recycled cellulose matrix improved membrane porosity (i.e., from 41% for pristine to 64%) and hydrophilic properties. The authors ascribed this to the increase in free volume between the cellulose chain and the superficial hydroxyl group presented on the TiO
2 surface, respectively, which subsequently enhanced the pure water flux (from 0.69 L m
−2 h
−1 for the pristine membrane to 4.12 L m
−2 h
−1 for the photocatalytic cellulose membrane with 0.5 wt% N-doped TiO
2 nanorods). The membrane also demonstrated the efficient photocatalytic degradation of aqueous phenol under visible light (78.8%) and ultraviolet irradiations (96.6%)
[53][140],
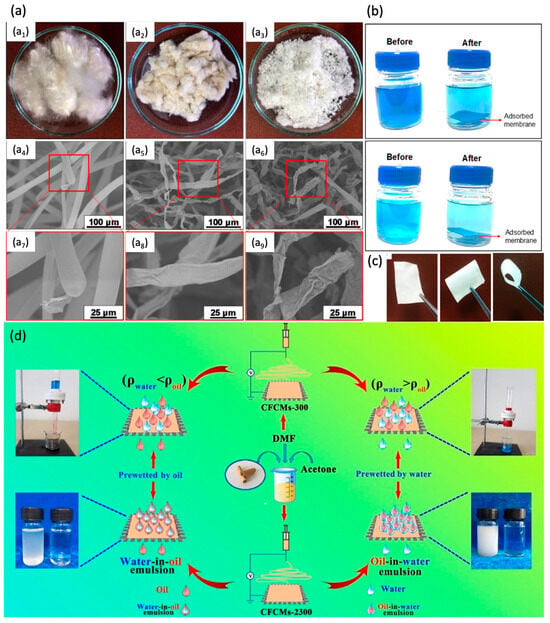
Using CA derived from the cigarette, an electrospun membrane for treating the oil/water emulsion was fabricated by Liu et al.
[51][138] by dissolving the collected waste cigarette filters in a solvent mixture (10.5 mL DMF and 3.4 mL acetone). The membrane exhibited superhydrophobicity (under oil) when prewetted with the heavy oil, thus facilitating the oil permeation (i.e., >99% efficiency with a filtrate flux of >100 L m
−2 h
−1) while blocking the water on the other side of the membrane (
Figure 610b). Doyan et al. also fabricated a CA (derived from a cigarette filter) membrane via phase inversion using DMF as a solvent to treat the oil/water emulsion
[54][141]. The membrane demonstrated considerable potential with a permeability of 180 L m
−2 h
−1 bar
−1 and a rejection rate exceeding 94% after undergoing five filtration cycles of the oil/water emulsion. Despite providing greater permeability to oil-in-water emulsions than PSF and PVDF membranes, the increased oil adhesion on the membrane surface caused the membranes to experience more severe irreversible fouling (>90.0%). The results indicated that, similar to the majority of CA membranes made from commercially available CA materials, it is necessary to optimize the membrane composition or modify the membrane surface in order to address the fouling problem.