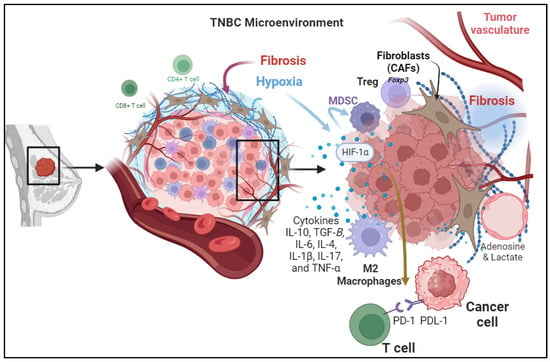
The intricate components contributing to the Tumor Microenvironment (TME) in Triple-Negative Breast Cancer (TNBC) are depicted. Cellular Components of the TME, highlighting the diverse cell types such as Tumor-Infiltrating Lymphocytes (TILs), Myeloid-Derived Suppressor Cells (MDSCs), and Tumor-Associated Macrophages (TAMs) and their roles in immunosuppression. Tumor-induced hypoxia, illustrating the impact of hypoxia on immune response through various pathways including immune checkpoint activation and myeloid cell recruitment. Secretion of Immunosuppressive Cytokines, outlining the role of cytokines, such as TGF-β, IL-10, IL-6, IL-4, IL-1β, IL-17, and TNF-α, in shaping the immunosuppressive microenvironment. Metabolic Reprogramming, showcases the shift in energy metabolism and the production of immunosuppressive metabolites such as adenosine and lactic acid, influencing immune cell function. Changes in the configuration of the Extracellular Matrix (ECM) emphasize the role of fibrosis in creating a barrier that hinders effective drug delivery and immune cell infiltration. Understanding these components is vital for developing targeted therapies to modulate the TME and enhance antitumor immune responses in TNBC patients.
In addition to the conventional mechanisms of immunosuppressive responses at the tumor site due to activation of immune checkpoint pathways including the PD-1/PD-L1 Axis and CTLA-4 Pathway, there are other factors involved. For instance, hypoxia commonly happens in rapidly growing tumors that outsource the available blood supply, resulting in hypoxia within the TME. Hypoxia induces expresses the Angiogenic Growth Factors, which are associated with the stabilization and activation of hypoxia-inducible factors (HIFs) which are transcription factors that regulate the expression of genes involved in various aspects of tumor progression, including immunosuppression
[16][19]. It can also promote the recruitment and expansion of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), both of which contribute to the immunosuppression
[17][20].
3.3. Secretion of Immunosuppressive Cytokines
Cytokines are small signaling proteins secreted by various cell types, including tumor cells, immune cells, and stromal cells, within the TME. These cytokines play a crucial role in modulating the immune response and influencing tumor progression. Different cytokines contribute to the suppression of immune response in the tumor microenvironment. These cytokines are secreted by cancer cells and stromal cells in the tumor microenvironment including immune cells, endothelial cells, and fibroblasts. For example, cytokines secreted by cancer-associated fibroblasts (CAFs) cause immunosuppression, tumor cell proliferation, and remodeling of the ECM.
TGF-β is a multifunctional cytokine with immunosuppressive properties. In the TNBC TME, TGF-β is often overexpressed and released by both tumor cells and immune cells
[18][23]. It has several immunosuppressive including the inhibition of T lymphocytes proliferation and activation of cytotoxic CD8+ T cells and NK cells, reducing their ability to target and kill tumor cells
[19][24].
Interleukin-10 (IL-10): IL-10 is an anti-inflammatory cytokine that is often elevated in the TNBC TME. Its immunosuppressive effects include suppressing the production of pro-inflammatory cytokines, such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), by immune cells
[20][26]. In addition, it inhibits the expression of major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs), reducing their ability to present tumor antigens to T cells
[21][27].
Interleukin-6 (IL-6): IL-6 is a pro-inflammatory cytokine that plays a dual role in cancer. In TNBC, it can have both pro- and anti-tumorigenic effects; IL-6 can promote the recruitment and activation of immune cells to the TME, potentially enhancing antitumor immune responses. However, IL-6 can also contribute to immunosuppression by inducing the differentiation of Tregs and promoting the expansion of MDSCs
[22][28].
Interleukin-4 (IL-4): IL-4 is an immunoregulatory cytokine that can contribute to immunosuppression in TNBC by promoting the polarization of macrophages toward the M2-like phenotype, which supports tumor growth and inhibits immune responses
[23][29].
Interleukin-1β (IL-1β): IL-1β is a pro-inflammatory cytokine produced by various cells within the TME, including tumor cells, immune cells, and stromal cells. In TNBC, IL-1β can promote tumor growth and immunosuppression by Inducing the production of other pro-inflammatory cytokines and chemokines, thereby contributing to a chronic inflammatory environment and regulating the differentiation and activity of immune cells, potentially promoting a more immunosuppressive phenotype
[24][30].
Interleukin-17 (IL-17): IL-17 is a cytokine primarily produced by a subset of T cells known as Th17 cells. In the TNBC TME, IL-17 can have dual roles It can stimulate the recruitment of immune cells to the TME, potentially enhancing antitumor immune responses
[25][31]. However, chronic IL-17 signaling may also contribute to tumor progression by promoting angiogenesis and tissue remodeling
[26][32].
Tumor Necrosis Factor-Alpha (TNF-α): TNF-α is a pro-inflammatory cytokine that can have both pro- and anti-tumor effects. In TNBC, it can promote tumor cell death and inhibit angiogenesis. However, chronic TNF-α signaling can also contribute to the recruitment of immunosuppressive cells like MDSCs and TAMs
[27][33].
3.4. Metabolic Reprogramming
Metabolic reprogramming, a hallmark of cancer cells, involves a shift in energy production and utilization to sustain the high proliferation rates observed in tumors
[28][34]. In TNBC, this metabolic switch often entails a preference for aerobic glycolysis, known as the Warburg effect
[29][35]. Beyond providing energy, these metabolic alterations play a pivotal role in establishing an immunosuppressive TME. One key aspect of metabolic reprogramming in TNBC is the increased production of metabolites that contribute to immunosuppression.
Moreover, altered amino acid metabolism in TNBC contributes to the generation of immunosuppressive signals
[30][37]. Cancer cells often upregulate enzymes involved in tryptophan metabolism, leading to the production of kynurenine. Elevated kynurenine levels have been linked to the inhibition of T cell function and the promotion of regulatory T cell (Treg) differentiation, both of which contribute to immune evasion in the TME
[30][37].
3.5. Changes in the Configuration of the Extracellular Matrix in the TME in TNBC
One prominent feature of the tumor microenvironment (TME) in TNBC is the development of fibrosis, which has recently garnered attention for its potential role in shaping immunosuppressive mechanisms. Fibrosis, the excessive deposition of extracellular matrix components, is commonly associated with chronic inflammation and tissue remodeling
[31][40]. In the context of TNBC, fibrosis contributes to the formation of a dense and fibrotic TME, creating a barrier that can impede effective drug delivery and immune cell infiltration
[32][41]. Paradoxically, this fibrotic response also plays a crucial role in the establishment of immunosuppressive mechanisms within the TME. The fibrotic TME in TNBC is characterized by the activation of cancer-associated fibroblasts (CAFs) and the increased deposition of proteins such as collagen and fibronectin. These changes create a physically and biochemically hostile environment, limiting the infiltration and activity of Tumor-infiltrating lymphocytes (TILs)
[33][42].
4. Overcoming Mechanisms of Immune Resistance within TME of TNBC Patients
4.1. Monotherapy-ICIs
Immune checkpoints, which are expressed on the surface of immune cells (IC) and tumor cells (TC), serve as a communication bridge between cells in the tumor immune microenvironment (TME). Immunogenic status within the TME reflects the communication status between cells. Recently, the blockade of immune checkpoints has been used to remodel the immune response in the TME. Hence, an ICI is a monoclonal antibody that targets a specific immune checkpoint, in which PD-1 is highly expressed on T, NK, and myeloid immune cells while PD-L1 is found either on tumor or activated macrophages, T cells, and CAFs
[1][34][1,44].
The United States Food and Drug Administration (FDA) granted approval in the year 2019 for the utilization of the PD-L1 inhibitor (Atezolizumab) in conjunction with chemotherapy (Abraxane) as a treatment for patients with metastatic TNBC
[35][45].
The initial stage of the KEYNOTE-012 clinical research investigation involved a cohort of 111 patients diagnosed with triple-negative breast cancer (TNBC). These patients received a PD-L1 inhibitor, specifically Pembrolizumab, administered intravenously at a dose of 10 mg/kg every two weeks.
Phase 1b JAVELIN solid tumor investigation employed a PD-L1 inhibitor known as Avelumab to manage patients with metastatic breast cancer, including 58 cases of triple-negative breast cancers (TNBCs). The Avelumab treatment resulted in a 5.3% objective response rate (ORR) in TNBCs. The ORR observed in TNBC patients who exhibited positivity for PD-L1 was determined to be 22.2%, whereas the ORR in PD-L1-negative patients was found to be 2.6%
[36][47].
PD-L1 is prominently exhibited in tumor cells and its interaction with PD1-immune cells is employed as a defensive mechanism against predominantly T cells. Furthermore, the notable expression of PD-L1 on malignant cells is linked to the dissemination of cancer cells and unfavorable prognosis in lymph nodes. As a result, a fresh immunotherapy strategy is being devised to explore the inherent impact of PD-L1 in TNBC without PD-1. In laboratory conditions, the investigation employed PD-L1-siRNA to mute the PD-L1
[37][48] and assess the cell proliferation, cell migration
[38][49], tumor apoptosis, and T cell induction to TMIE.
4.2. Dual ICIs
PD-1/PD-L1 inhibition during the initial phases in patients with triple-negative breast cancer resulted in a response rate ranging from 18% to 24%
[39][50]. As of 2023, the coadministration of Pembrolizumab, a monoclonal antibody targeting the programmed cell death protein 1 (PD-1), and Doxorubicin in individuals with anthracycline-naïve metastatic triple-negative breast cancer (mTNBC) has displayed a synergistic effect in stimulating the cellular immune response and managing the disease. It is noteworthy that a significant 67% of mTNBC patients exhibited an overall response to the treatment, accompanied by a notable activation of T cells
[40][51].
Using either anti-PD-1 or anti-CTLA4 as monotherapy has demonstrated a limited immune response against tumors. However, the combination of anti-PD-1 and anti-CTLA4 in the animal model of TNBCs enhances the effectiveness of anti-PD-1 and anti-CTLA4 against TNBC.
4.3. Chemotherapy Combined ICIs
Striking success has been achieved in cancer outcomes and immune resistance via combining ICIs with specific chemotherapy. Particularly, platinum-based chemotherapy promoted ICI efficacy by making tumor cells more sensitive to PD1/PD-L1 inhibitors and highly expressed to PD-L1
[41][57]. Recent Phase III clinical trials conducted on patients diagnosed with triple-negative breast cancer have provided evidence to support the effectiveness of combining chemotherapy with immunotherapy, specifically ICIs as a potential treatment option
[42][58].
New strategies are currently focused on the inhibition of angiogenesis within the tumor microenvironment (TME), which creates a barrier against the infiltration of CD8+ T cells into the TME. Despite the collaborative efforts of immune cells to disrupt tumor vessels, particularly through the activity of IFNG-secreting CD8+ T cells and M2 macrophages, tumor angiogenesis still impacts the infiltration of CD8+ T cells.
4.4. Cancer Vaccine Combined ICIs
The utilization of CTLA4 blockade remains restricted and the prognosis of cancer can be controlled in specific subtypes of triple-negative breast cancer (TNBC). Interestingly, recent data suggests that the inclusion of a CTLA4 inhibitor in conjunction with a MUC1 mRNA nanovaccine enhances the therapeutic impact of the anti-CTLA-4 monoclonal antibody and adjusts the immune resistance within the tumor immune microenvironment (TME)
[43][63]. Both preclinical and clinical investigations ensure the remarkable nature of a therapeutic combination. Thus far, the immune-modifying function of a CTLA4 inhibitor combined with an MUC1 mRNA nanovaccine in the tumor microenvironment (TME) of triple-negative breast cancer (TNBC) has been evaluated by examining the cytotoxic immune cells and cytokines. It is worth noting that the combination of anti-CTLA4 and MUC1 mRNA vaccine resulted in an increased presence of cytotoxic CD8+ T cells and elevated levels of IL12 and IFN gamma.
4.5. Combining ICIs with TME Metabolites
In actuality, the tumor microenvironment is primarily characterized by a multitude of metabolic disturbances. The presence of cancer cells is associated with a state of accelerated metabolism, whereby metabolites play a pivotal role in facilitating tumor cell proliferation, migration, and maturation. An illustrative example of this phenomenon involves the non-essential amino acid, glutamine, which serves as a valuable nutritional resource for tumor cells.
Profoundly, overexpression of immune checkpoints in tumor cells is accompanied by an increase in metabolite production which provokes immune suppression such as lactate and glycolysis.
4.6. Cytokines–IFN/TGF Beta Crosstalk with ICIs
Breast cancer cells often use immune checkpoint blockade to evade the immune system’s response, as they have high levels of PD-L1 which helps them create anti-immune responses. To enhance immune response, blocking immune checkpoints is the best approach, with dual blockade being the most rational for TNBC. The regulation of PD-L1 expression can be achieved at the transcriptional or post-transcriptional level, and IFN-γ serves as a crucial regulator for PD-L1 in the tumor microenvironment (TME) of TNBC. It is worth noting that IFN-γ exhibits the remarkable ability to impede tumorigenesis by inducing apoptosis in tumor cells and inhibiting angiogenesis.
The use of ICIs in treating triple-negative breast cancer (TNBC) remains ineffective due to multiple factors, for instance, the presence of Transforming Growth Factor (TGF-β), which is known to reprogram the tumor microenvironment in Triple Negative Breast Cancer. It suppresses the immune system by increasing collagen production in cancer-associated fibroblasts.
4.7. Anti CD25/Anti CD47 Combined with ICIs
The imbalance of immune cells within the TME’s TNBC drew the researcher’s interest in revealing the most prominent cell types within all stages of TNBC and understanding their impacts on TME. Consequently, they have discovered that the most prevalent cell types in primary TNBC patients are CD25
high effector regulatory T cells (eTregs), while effector T cells (Cytotoxic CD8+) are significantly diminished. Furthermore, the abundance of eTregs is linked to resistance against anti-PD-1 therapy in TNBC.
4.8. Chemokine Inhibitors Cross-Talk with ICIs
Genomic analysis has revealed that TNBCs TME exhibits elevated expression of CXCR4 and its ligand, SDF-1 (also known as CXCL12). Both primary TNBCs and their metastatic forms display high levels of the G-protein-coupled receptor CXCR4. The CXCL12/CXCR4 axis attracts immune suppressive cells to TME, including M2-phenotype macrophages, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs).
4.9. Gut Microbiota Crosstalk with ICIs
Earlier investigations showed the gut microbiota as a critical player in most human diseases. Hence, host gut microbiota cross-talks various physiological processes via modulating immune response, epithelial production, and metabolite production to keep hemostasis. Interestingly, in cancer diseases, human gut microbiota work as immunosuppressive and oncogenic actors based on the abundance of microbiota type and other factors
[44][71].
Hence, the examination conducted by Wang and colleagues (2022) has primarily concentrated on comprehending the tumor microenvironment (TME) of triple-negative breast cancer (TNBC) patients. Within this context, the researchers have observed the prevalence of
Clostridiales and metabolite trimethylamine N-oxide (TMAO) within the TME of TNBC. TMAO and
Clostridiales have exhibited a positive correlation with TNBC patients undergoing immunotherapy
[45][73].