Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Miquéias Lopes-Pacheco.
Sepsis and acute respiratory distress syndrome (ARDS) are complex and heterogeneous syndromes for which no specific therapies exist. Mesenchymal stromal cell (MSC) administration significantly reduces tissue inflammation and remodeling, improves pathogen clearance, and reduces morbidity and mortality in multiple preclinical models of sepsis and acute lung injury (ALI, the animal corollary to ARDS)
- acute lung injury
- acute respiratory distress syndrome
- extracellular vesicles
- exosomes
- mesenchymal stromal cells
- microRNA
- nano therapy
- sepsis
1. Introduction
Sepsis and acute respiratory distress syndrome (ARDS) are complex and heterogeneous syndromes for which no specific therapies exist. Mesenchymal stromal cell (MSC) administration significantly reduces tissue inflammation and remodeling, improves pathogen clearance, and reduces morbidity and mortality in multiple preclinical models of sepsis and acute lung injury (ALI, the animal corollary to ARDS) [1]. Significant morbidity and mortality associated with both syndromes, as well as the lack of specific therapies, explains the urgency in demonstrating the therapeutic benefit of allogenic delivery of MSCs in the clinic. Although multiple studies have identified various targets that, when pharmacologically or interventionally altered, mitigate various pathological features of sepsis and ARDS experimentally (in vitro and in vivo) and in Phase I and II clinical trials, none has yielded effective treatments in Phase III randomized clinical trials.
Since the initial isolation and culture of human MSCs over 1300 registered clinical trials (clinicaltrials.gov “mesenchymal” 6/5/20) [2] have demonstrated the safety of administering these cells in humans [3]. Notwithstanding, we have yet to replicate the large effect sizes predicted from pre-clinical research [4,5][4][5], as small and large trials have failed to meet efficacy endpoints [6]. Inconsistent results have been attributed to product heterogeneity and irregularities, use of pre-clinical models that may not recapitulate the complexities of human disease, transferability across species, and/or poor estimation of effect size from preclinical data leading to inconclusive findings in humans [2,7][2][7]. Moreover, although the potential of MSCs remains undisputed, questions remain concerning their mechanisms of action (MoA).
2. MSCs Are Not Required for Therapeutic Effect in Sepsis/ALI
In preclinical studies of sepsis and ALI, MSCs delivered through a variety of different routes moderate multiple organ failure and reduce mortality [1]. From the initial studies, various investigators argued that although the precise MoA remains to be elucidated, cell engraftment with differentiation, transdifferentiation, or cell fusion were unlikely to contribute to observed beneficial effects in preclinical models of sepsis/ALI. First, very low levels of cell persistence could be demonstrated after infusion. Following intravenous administration, MSCs are efficiently targeted to the lung where they are trapped simply by size-based filtration in the distal pulmonary arteriolar bed [10][8]. Using highly sensitive methods (i.e., quantitative real-time polymerase chain reaction (RT-PCR) for the sex-determining region Y protein in female recipients [10,11,12][8][9][10]) or fluorescent labeling approaches [13,14[11][12][13],15], cells seem to be present in the lung only up to 28–96 h post-infusion. Second, whole cells are not required for their therapeutic effect. Over a decade ago, it became apparent that the cultured supernatant from MSCs can mitigate lung injury by providing similar protection to that observed with viable whole cells [22[14][15],23], even when aerosolized [24][16]. In addition to confirming cell homing is not necessary for imparting therapeutic effects [25][17], this finding strongly supported a paracrine MoA. Multiple studies have also convincingly demonstrated that the immunomodulatory effect of MSCs is communicated via MSC and recipient-secreted cytokines and relies on the local microenvironment [19[18][19][20][21][22],26,27,28,29], as some of the observed effects depend on a pre-treatment of MSCs with inflammatory cytokines [27,28,30][20][21][23]. More recent findings indicate that the cytokine-mediated effects are only one part of the equation, as MSC-derived microparticles (including exosomes), apoptotic, metabolically inactivated, or even fragmented MSCs and MSC-membranes have immunomodulatory potential [31,32,33,34,35][24][25][26][27][28]. These observations have raised important questions regarding the putative direct targets of MSCs and the active therapeutic “ingredients” contained in an MSC, its secretome-derived “soup” or/and -derived microparticle/fragments, that confer their beneficial effects.3. MSC-Derived Secretome
A large portion of MSC’s therapeutic activity is attributed to direct primary signaling through their secretome, comprising a multitude of cytokines, chemokines, growth factors, and subcellular vesicles; overviews of the current state of knowledge of MSC’s secreted mediators and how inflammatory priming influences their release have been published [36,37,38][29][30][31]. Target identification studies have demonstrated that MSCs have a wide pleiotropic effect on the pathophysiology of complex syndromes, specifically sepsis and ALI. OuThe researchers' group has previously published—using a model of polymicrobial sepsis induced by cecal ligation and puncture (CLP)—that MSC administration alters the expression of 3968 genes in five different sepsis-target organs (lung, liver, spleen, kidneys, and heart) [39][32]. Given about 30,000 as the estimated total number of genes in the mouse genome, ourthe data suggest that approximately 13% of the septic genome is transcriptionally reprogrammed after MSC administration [39][32]. While multiple gain- (overexpressing) and loss- (silencing)-of-function studies have identified key mediators involved in the therapeutic effect, including IL-10 [40][33] and anti-bacterial peptide LL-37 [41][34], none have emerged as the single most responsible therapeutic target. The release of EVs in the secretome represents an immediate cell communication mechanism that fulfills major criteria to impart MSC-related functions: (a) dissemination of membrane-bound mediators/signaling molecules; (b) transfer of expression patterns from one cell to another; and (c) rapid rearrangement of the cell surface. Like all messengers, EVs can not only immediately modify a phenotype of neighboring cells in a paracrine fashion but also bring forward an altered micro-milieu to other tissues by transferring the membrane composition and/or expression pattern to distal organs [42][35]. Salutary as well as pathological effects may be imparted by information contained on EVs and their cargo in the form of circulating nucleic acids (mRNAs and miRs), lipids, and proteins [31,43,44][24][36][37]. Seminal work from Monsel and colleagues demonstrated the therapeutic effects of human MSC-derived EVs in a mouse model of severe pneumonia [45][38]. In this model, the group was able to show that EV delivery significantly enhanced the expression of keratinocyte growth factor (KGF) in recipients associated with improved survival. A subsequent clinical trial randomized patients with ARDS to receive recombinant KGF or placebo. The trial was stopped early because of increased harm in the group that received KGF [46][39]. While multiple reasons are usually linked to unsuccessful clinical trials, results did suggest that the increase in KGF is a meta-phenomenon rather than directly causal to the improved mortality seen in animal models. Herein lies the difficulty and the critical importance of understanding and translating promising preclinical data to the clinic. Together, these data challenge the paradigm that a single, specific MSC-derived paracrine mediator is responsible for the global pleiotropic effect of MSCs on transcriptional and network reprogramming in sepsis. A much more plausible explanation is that MSC-conferred protection from sepsis includes a range of complementary activities, resulting in the mitigation of the innate and acquired immune and inflammatory responses. Of particular interest to outhe researchers' group is the potential therapeutic role of regulatory RNAs contained within EVs. Below, wethe researchers reviewed the literature implicating epigenetic regulatory small RNAs, specifically miRs, as important regulators and mediators of the therapeutic effect of MSC-derived EVs.4. MSC-EV Content
MSC-derived EVs act as paracrine mediators of MSCs’ beneficial effects. In a landmark publication [47][40], Phinney and colleagues demonstrated that MSCs are inefficient in performing mitophagy; using microvesicles to shuttle mitochondrial components out of the cell. As MSCs outsource mitophagy to recipient macrophages [47][40], they simultaneously package anti-inflammatory miRs into smaller EVs (including microRNA let-7f, c and I, 23b, 27b, and 29a). These miRs were shown by the group to inhibit critical pattern and damage recognition receptors and signaling molecules: Toll-like receptor (TLR)4, MyD88, TLR9, TLR7, and tumor necrosis factor-alpha (TNF-α) in recipient macrophages. There is little doubt EVs contain anti-inflammatory miRs, that are produced and released by MSCs; what is unclear is why the cells would do this, and whether these miRs are actually therapeutically active? Much of the controversy around which component of EV payload is biologically active (contributes to MoA) comes from non-standardized EV preparations. The importance of this issue cannot be understated [58,59,60][41][42][43]. The International Society for EVs has established clear criteria for isolating, characterizing, and defining microparticles derived from MSCs [61,62][44][45]. Moreover, Dr. Sai Kiang Lim and colleagues have recently published criteria for defining MSC-derived EVs for therapeutic application, including establishing MSC origin (concentration of CD73, CD90, and CD105; and absence of non-MSC antigens CD14, CD34, and CD11b); number of particles per unit weight protein/membrane lipids and particle diameter (within 50–200 nm); molar/weight ratio of protein to membrane lipids (e.g., cholesterol and phosphatidylcholine); and presence of biochemically active cargo (enzyme activity of CD73, unit activity per μg protein) [63][46]. The fact that MSC-derived EVs and miRs are therapeutically relevant is not disputed; the issue is whether the miRNAs that are carried inside EVs are actually the ones imparting their beneficial effects.5. EV-microRNAome
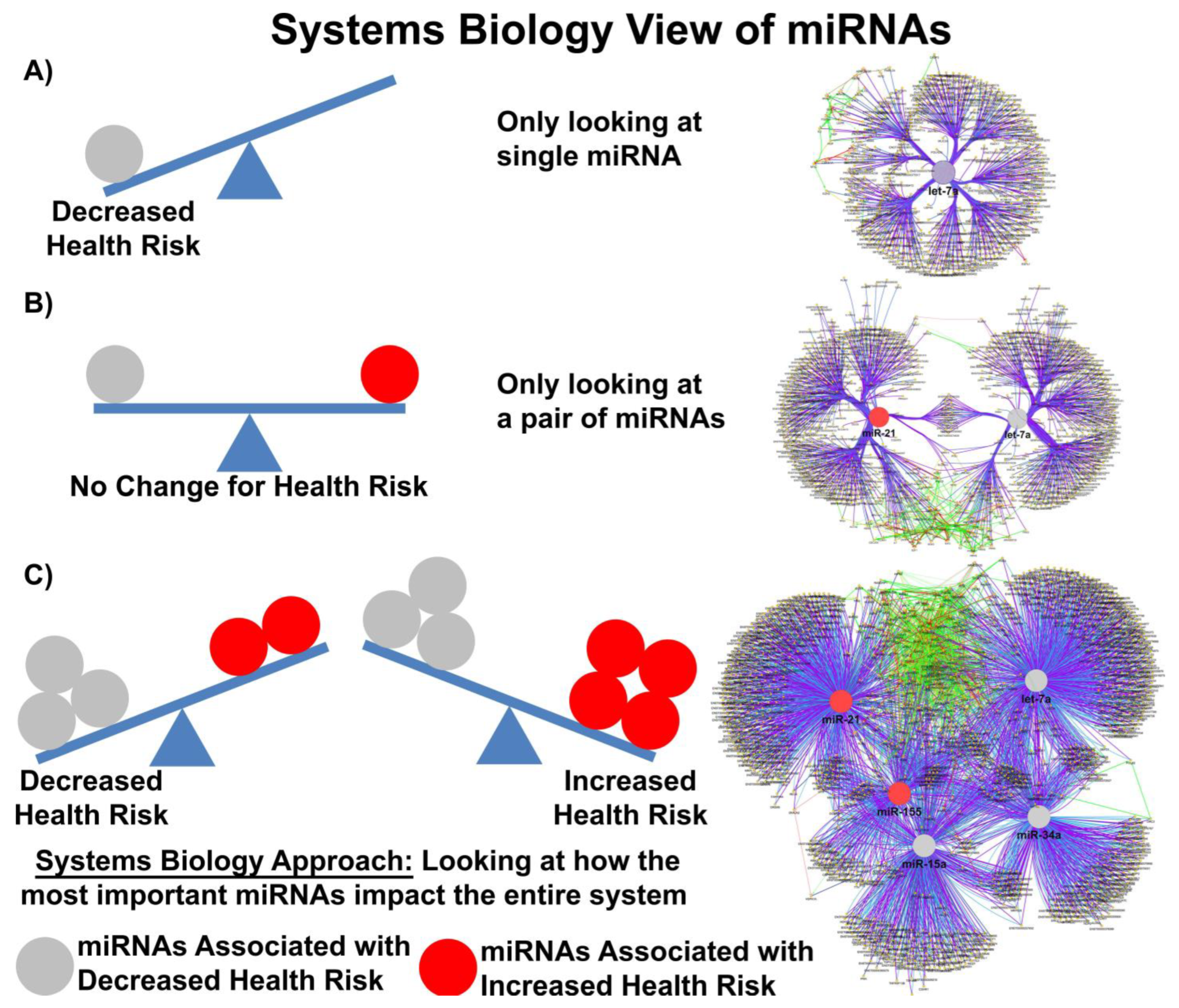
In the publication from Valadi and others, the authors determined that each exosome may carry as many as 100–120 different miRs and that some miRs were expressed to a greater extent in exosomes compared to donor cells [48][47]. Baglio and colleagues further demonstrated that MSCs of different origins have similar small RNA expression profiles; EV-derived miRs (EV-miRs) or exo-miRs represent about 1–2% of the cellular RNA content, and multiple highly expressed miRs are precluded from EV sorting [64][48], suggesting that the process of packaging miRs into EVs is highly regulated and not simply a mechanism for managing cellular waste. Quantitative and stoichiometric analysis of the miR content of EVs demonstrated less than one copy of each microRNA per EV [65][49]. Using ultrasensitive total internal reflection-based single-vesicle in situ quantitative and stoichiometric analysis of tumor-derived EV miRs, He and colleagues demonstrated that each EV may contain as many as 6 copies of different unique microRNAs [66][50]. But what is the functional dose of a miR? Based on the literature, the functional dose may differ for each target [67][51]; with factors such as the expression levels of the targets, the number of miR-binding sites on the target mRNA and feedback loops able to shift this narrow window for effective gene suppression. MiRs may also repress their targets in a nonlinear manner introducing thresholds in gene expression [68][52]. Furthermore, as miRs can work cooperatively or competitively [69][53], dose-dependent mRNA target selection becomes even more complicated when combinations of miRs, with potentially overlapping targets, are taken into account, such as would happen in EVs carrying a variety of different miRs. Electroporation nano straw delivery, which can bypass biological mechanisms, has been used to deliver miRs with precise dosage control directly into primary cells [70][54]. An example of the importance of dose comes from studies using miR-17-92, which was shown to decrease the cell viability of colon cancer cell line at low doses (0.00003 μg plasmid) but increase cell viability at high doses (0.3 μg plasmid) [67][51]. MiR fold changes as low as 3–4 are sufficient to drive the development of disease in transgenic mice [71][55], and a 1.5-fold change in the expression level of a single miR is considered enough to alter phenotype. Denzler and colleagues showed that when a miR is lowly expressed, only the highest-affinity sites are sufficiently occupied to mediate repression, but as miR expression increases, more and more intermediate and low-affinity sites have occupancies sufficient to mediate repression [72][56]. Canonical 6-nucleotide (-nt) sites, which typically mediate modest repression, can nonetheless compete for miR binding, with a potency of 20% of that observed for canonical 8-nt sites. This suggests there is a strong relationship between site affinity and miR-dose that is significantly impacted by competition between different miRs for overlapping binding sites. Moreover, cooperative binding of proximal sites for the same or different miRs does increase potency, making it very difficult to understand the relationship between dose and biological effect. In most clinical studies, EV doses range between 1–10 × 108–12 particles/mL, suggesting that while the copy number inside an EV may be small, the EV dose may deliver a significant number of therapeutically relevant miRs. Moreover, given that a single miR can regulate the expression of hundreds of genes, a system biologist’s view of miR networks suggests that the biological effects of a single functioning miR may significantly amplify the biological effect at a systems level (Figure 1) [73][57].
Figure 1. Systems biology view of microRNA Networks. Figure generated with Cytoscape and ClueGO by Dr. Afshin Beheshti, Bioinformatician and Principal Investigator, Blue Marble Space Institute of Science, NASA Ames Research Center, Space Biosciences Research Branch, reproduced with permission from Dr. Beheshti and NASA Ames Research Center.
6. Evidence That miRs Transferred in MSC-Derived EV Are Functional
Accumulating evidence suggests that miR transfer in MSC-derived EVs plays an important role in attenuating sepsis-associated lung injury by exerting anti-inflammatory and anti-apoptotic effects in both in vitro and in vivo models of lung disease. Specifically, in models of septic and non-septic ALI, several miRs (miR-21-5p, miR-27a-3p, miR-30b-3p, miR-100, miR-384-5p, miR-145, miR-146, miR-125b-5p, and miR-181a-5p, among others) transferred in MSC-derived EVs have been identified to be involved in therapeutic actions. Macrophages are key innate immune cells involved in ARDS inflammatory response, and many studies have investigated the ability of EVs to modulate macrophage inflammatory state. MiR-145 has been shown to account for the human bone marrow MSC-derived EV effects in the model of E.coli-induced lung injury. It has been demonstrated that miR-145 packaged in EVs is transferred into murine macrophages to enhance macrophage phagocytosis and to reduce E. coli bacterial load through increasing leukotriene B4 levels [74][58]. Moreover, miR-145 is also responsible for modulating ABCC1, an ATP-binding cassette multidrug resistance protein [74][58]. Song and colleagues found that miR-146a was strongly upregulated in MSCs by IL-1β licensing and selectively packaged into EVs. This group showed that miR-146a was transferred to macrophages, resulting in M2 polarization leading to increased survival in septic mice [75][59]. Inhibition of miR-146a in MSCs partially negated the immunomodulatory properties of MSC-derived EVs [75][59]. Similarly, Yao and colleagues demonstrated that macrophages are polarized into M2 phenotype by miR-21 transferred from MSC-derived EVs pre-treated with IL-1β, which significantly enhanced the therapeutic effects in experimental sepsis [76][60]. These macrophages demonstrated decreased production of IL-8 and TNF-α, increased expression of IL-10, and enhanced phagocytosis activity [77,78][61][62]. In the mouse model of ischemia/reperfusion lung injury, the anti-apoptotic miR-21-5p in MSC-derived EVs was transported to alveolar macrophages, contributing to macrophage reprogramming toward M2 macrophages via phosphatase and tensin homolog (PTEN) and programmed cell death protein 4 (PDCD4) [79][63]. Such effects were reversed when MSCs were pretreated with miR-21-5p antagonists, resulting in persistent apoptosis of pulmonary endothelial cells [79][63]. Wang and colleagues have shown that miR-27a-3p packaged in MSC-derived EVs was taken up by alveolar macrophages and directly targeted nuclear factor kappa B (NF-κB) subunit 1, thereby downregulating NF-kappa B signaling and promoting M2 macrophage polarization in the murine LPS-induced model of lung injury [80][64]. Lentiviral transduction of MSCs with anti-miR-27a-3p or knockdown of miR-27a-3p in vivo abolished the therapeutic effects of MSC-derived EVs in that model [80][64]. Ferroptosis is a unique modality of cell death driven by iron-dependent phospholipid peroxidation. Shen and colleagues reported that adipose tissue MSC-derived EV transfer of miR-125b-5p rescues pulmonary microvascular endothelial cells ferroptosis and improves survival in a CLP model of sepsis. Such effects were mediated via the Keap1/Nrf2/GPX4 pathway [81][65]. Furthermore, Pei and colleagues recently reported that human-umbilical-cord-MSC-derived EVs prevent inflammation and reduce the severity of lung injury in the mouse model of sulfur mustard-induced lung injury through the delivery of miR-146a-5p [82][66]. In particular, they showed that miR-146a-5p expression levels were markedly decreased after injury, while it was restored in lung tissue after treatment with MSC-derived EVs. Moreover, through a series of elegant experiments, the authors deciphered that miR-146 targeted TRAF6 and mediated the anti-inflammatory effect through modulation of TLR4, TRAF6, IRAK1, and NF-κB pathways. Overexpression of miR-146a-5p in MSC-derived EVs significantly attenuated TRAF6 expression, which negatively regulated sulfur mustard-induced inflammation in vitro and in vivo [82][66]. MiR-451 is another factor that is highly abundant in MSC-EVs [83][67]. This miR was found to inhibit the expression of IL-1β, IL-6, and TNF-α by suppressing the TLR4/NF-κB signaling pathway in burn-induced ARDS. Such effects were abrogated when miR-451 expression was suppressed [83][67]. Furthermore, both miR-23a-3p and miR-182-5p carried by MSC-EVs were able to reverse LPS-induced injury and fibrosis by silencing Ikbkb and destabilizing IKKβ, which prevented the downstream activation of NF-κB and hedgehog pathways [84][68]. MSC-derived EVs overexpressing miR-30b-3p were protective in the mouse model of LPS-induced lung injury presumably through inhibition of serum amyloid A3 (SAA3). This factor is a major component of the acute phase of inflammation and is a direct target of miR30b-3p. In MLE-12 cells (mouse alveolar type II epithelial cell line), miR30b-3p transferred from MSC-derived EVs protected cells against LPS-induced injury and enhanced cellular proliferation [85][69]. Furthermore, miR-132-3p carried by MSC-derived EVs reduced LPS-induced injury in mouse lung epithelial cells by targeting TRAF6, inhibiting PI3K/Akt signaling and enhancing cellular proliferation [86][70]. In endothelial cells, MSC-EV-derived miR-126 induced downregulation of the Sprouty-related EVH1 domain-containing protein 1 (Spread-1), which prevented LPS-induced injury and enhanced cell function [87][71]. MiR-126 was also involved in inhibiting the expression of the alarmin HMGB1, which increased the expression of tight junction proteins [88][72]. miR-150 transferred from MSC-derived EVs was also able to mitigate LPS-induced endothelial injury by regulating caspase-3, Bax-Bcl-2, and MAPK signaling [89][73]. When miR-150 antagomirs were transfected into MSCs, these immunomodulatory effects were partly reversed [89][73].References
- Lopes-Pacheco, M.; Robba, C.; Rocco, P.R.M.; Pelosi, P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol. Toxicol. 2020, 36, 83–102.
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717.
- Thompson, M.; Mei, S.H.J.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 19, 100249.
- Lalu, M.M.; Sullivan, K.J.; Mei, S.H.; Moher, D.; Straus, A.; Fergusson, D.A.; Stewart, D.J.; Jazi, M.; MacLeod, M.; Winston, B.; et al. Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta-analyses prior to initiating a first-in-human trial. eLife 2016, 5, e17850.
- McIntyre, L.A.; Moher, D.; Fergusson, D.A.; Sullivan, K.J.; Mei, S.H.J.; Lalu, M.; Marshall, J.; McLeod, M.; Griffin, G.; Grimshaw, J.; et al. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: A systematic review. PLoS ONE 2016, 11, e0147170.
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833.
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24.
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057.
- Silva, J.D.; Paredes, B.D.; Araújo, I.M.; Lopes-Pacheco, M.; Oliveira, M.V.; Suhett, G.D.; Faccioli, L.A.P.; Assis, E.; Castro-Faria-Neto, H.C.; Goldenberg, R.C.S.; et al. Effects of bone marrow-derived mononuclear cells from healthy or acute respiratory distress syndrome donors on recipient lung-injured Mice. Crit. Care Med. 2014, 42, e510–e524.
- Lopes-Pacheco, M.; Ventura, T.G.; de Oliveira, H.D.; Monção-Ribeiro, L.C.; Gutfilen, B.; de Souza, S.A.L.; Rocco, P.R.M.; Borojevic, R.; Morales, M.M.; Takiya, C.M. Infusion of Bone Marrow Mononuclear Cells Reduces Lung Fibrosis but Not Inflammation in the Late Stages of Murine Silicosis. PLoS ONE 2014, 9, e109982.
- Carty, F.; Corbett, J.M.; Cunha, J.P.M.C.M.; Reading, J.L.; Tree, T.I.M.; Ting, A.E.; Stubblefield, S.R.; English, K. Multipotent Adult Progenitor Cells Suppress T Cell Activation in In Vivo Models of Homeostatic Proliferation in a Prostaglandin E2-Dependent Manner. Front. Immunol. 2018, 9, 645.
- Carty, F.; Dunbar, H.; Hawthorne, I.J.; Ting, A.E.; Stubblefield, S.R.; Van’t Hof, W.; English, K. IFN-γ and PPARδ Influence the Efficacy and Retention of Multipotent Adult Progenitor Cells in Graft vs Host Disease. Stem Cells Transl. Med. 2021, 10, 1561–1574.
- De Oliveira, H.D.; De Melo, E.B.B.; Silva, J.D.; Kitoko, J.Z.; Gutfilen, B.; Barboza, T.; De Souza, S.A.L.; Takiya, C.M.; Rocco, P.R.M.; Lopes-Pacheco, M.; et al. Therapeutic effects of bone marrow-derived mononuclear cells from healthy or silicotic donors on recipient silicosis mice. Stem Cell Res. Ther. 2017, 8, 259.
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389.
- Zhou, Z.; Hua, Y.; Ding, Y.; Hou, Y.; Yu, T.; Cui, Y.; Nie, H. Conditioned Medium of Bone Marrow Mesenchymal Stem Cells Involved in Acute Lung Injury by Regulating Epithelial Sodium Channels via miR-34c. Front. Bioeng. Biotechnol. 2021, 9, 640116.
- McCarthy, S.D.; Horgan, E.; Ali, A.; Masterson, C.; Laffey, J.G.; MacLoughlin, R.; O’Toole, D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Antibacterial Properties Against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 140–152.
- Hwang, B.; Liles, W.C.; Waworuntu, R.; Mulligan, M.S. Pretreatment with bone marrow–derived mesenchymal stromal cell–conditioned media confers pulmonary ischemic tolerance. J. Thorac. Cardiovasc. Surg. 2016, 151, 841–849.
- Park, J.; Kim, S.; Lim, H.; Liu, A.; Hu, S.; Lee, J.H.; Zhuo, H.; Hao, Q.; Matthay, M.A.; Lee, J.W. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 2019, 74, 43–50.
- Dunbar, H.; Weiss, D.J.; Rolandsson Enes, S.; Laffey, J.G.; English, K. The Inflammatory Lung Microenvironment; a Key Mediator in MSC Licensing. Cells 2021, 10, 2982.
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725.
- Lopes-Pacheco, M.; Rocco, P.R.M. Functional enhancement strategies to potentiate the therapeutic properties of mesenchymal stromal cells for respiratory diseases. Front. Pharmacol. 2023, 14, 1067422.
- de Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. 2019, 97, 605–618.
- Tindle, C.; Fuller, M.; Fonseca, A.; Taheri, S.; Ibeawuchi, S.-R.; Beutler, N.; Katkar, G.D.; Claire, A.; Castillo, V.; Hernandez, M.; et al. Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. eLife 2021, 10, e66417.
- Matthay, M.A. Extracellular Vesicle Transfer from Mesenchymal Stromal Cells Modulates Macrophage Function in Acute Lung Injury. Basic Science and Clinical Implications. Am. J. Respir. Crit. Care Med. 2017, 196, 1234–1236.
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transplant. 2015, 15, 2404–2412.
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191.
- Abreu, S.C.; Xisto, D.G.; de Oliveira, T.B.; Blanco, N.G.; de Castro, L.L.; Kitoko, J.Z.; Olsen, P.C.; Lopes-Pacheco, M.; Morales, M.M.; Weiss, D.J.; et al. Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma. Stem Cells Transl. Med. 2019, 8, 301–312.
- de Carvalho, L.R.P.; Abreu, S.C.; de Castro, L.L.; Andrade da Silva, L.H.; Silva, P.M.; Vieira, J.B.; Santos, R.T.; Cabral, M.R.; Khoury, M.; Weiss, D.J.; et al. Mitochondria-Rich Fraction Isolated From Mesenchymal Stromal Cells Reduces Lung and Distal Organ Injury in Experimental Sepsis. Crit. Care Med. 2021, 49, e880–e890.
- Moll, G.; Hoogduijn, M.J.; Ankrum, J.A. Editorial: Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front. Immunol. 2020, 11, 243.
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837.
- Abreu, S.C.; Lopes-Pacheco, M.; Weiss, D.J.; Rocco, P.R.M. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Perspectives. Front. Cell Dev. Biol. 2021, 9, 600711.
- dos Santos, C.C.; Murthy, S.; Hu, P.; Shan, Y.; Haitsma, J.J.; Mei, S.H.J.; Stewart, D.J.; Liles, W.C. Network Analysis of Transcriptional Responses Induced by Mesenchymal Stem Cell Treatment of Experimental Sepsis. Am. J. Pathol. 2012, 181, 1681–1692.
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49.
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238.
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Silva, J.D.; de Castro, L.L.; Braga, C.L.; Oliveira, G.P.; Trivelin, S.A.; Barbosa-Junior, C.M.; Morales, M.M.; dos Santos, C.C.; Weiss, D.J.; Lopes-Pacheco, M.; et al. Mesenchymal Stromal Cells Are More Effective Than Their Extracellular Vesicles at Reducing Lung Injury Regardless of Acute Respiratory Distress Syndrome Etiology. Stem Cells Int. 2019, 2019, 8262849.
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204.
- Monsel, A.; Zhu, Y.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell–derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336.
- McAuley, D.F.; Cross, L.M.; Hamid, U.; Gardner, E.; Elborn, J.S.; Cullen, K.M.; Dushianthan, A.; Grocott, M.P.; Matthay, M.A.; O’Kane, C.M. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir. Med. 2017, 5, 484–491.
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472.
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997.
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 590470.
- Bauer, F.N.; Giebel, B. CHAPTER 1. Therapeutic Potential of Mesenchymal Stromal Cell-derived Small Extracellular Vesicles. In Extracellular Vesicles: Applications to Regenerative Medicine, Therapeutics and Diagnostics; Royal Society of Chemistry: London, UK, 2021; pp. 1–21.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Raeven, P.; Zipperle, J.; Drechsler, S. Extracellular Vesicles as Markers and Mediators in Sepsis. Theranostics 2018, 8, 3348–3365.
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206.
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Pérez Lanzón, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.M.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127.
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893.
- He, D.; Wang, H.; Ho, S.-L.; Chan, H.-N.; Hai, L.; He, X.; Wang, K.; Li, H.-W. Total internal reflection-based single-vesicle in situ quantitative and stoichiometric analysis of tumor-derived exosomal microRNAs for diagnosis and treatment monitoring. Theranostics 2019, 9, 4494–4507.
- Shu, J.; Xia, Z.; Li, L.; Liang, E.T.; Slipek, N.; Shen, D.; Foo, J.; Subramanian, S.; Steer, C.J. Dose-dependent differential mRNA target selection and regulation by let-7a-7f and miR-17-92 cluster microRNAs. RNA Biol. 2012, 9, 1275–1287.
- Mukherji, S.; Ebert, M.S.; Zheng, G.X.Y.; Tsang, J.S.; Sharp, P.A.; van Oudenaarden, A. MicroRNAs can generate thresholds in target gene expression. Nat. Genet. 2011, 43, 854–859.
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37.
- Pop, M.A.; Almquist, B.D. Controlled Delivery of MicroRNAs into Primary Cells Using Nanostraw Technology. Adv. NanoBiomed Res. 2021, 1, 2000061.
- Jin, H.Y.; Oda, H.; Lai, M.; Skalsky, R.L.; Bethel, K.; Shepherd, J.; Kang, S.G.; Liu, W.-H.; Sabouri-Ghomi, M.; Cullen, B.R.; et al. MicroRNA-17~92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 2013, 32, 2377–2391.
- Denzler, R.; McGeary, S.E.; Title, A.C.; Agarwal, V.; Bartel, D.P.; Stoffel, M. Impact of MicroRNA Levels, Target-Site Complementarity, and Cooperativity on Competing Endogenous RNA-Regulated Gene Expression. Mol. Cell 2016, 64, 565–579.
- Vanderburg, C.; Beheshti, A. MicroRNAs (miRNAs), the Final Frontier: The Hidden Master Regulators Impacting Biological Response in All Organisms Due to Spaceflight. Available online: https://three.jsc.nasa.gov/Encyclopedia/Article/80 (accessed on 30 October 2023).
- Hao, Q.; Gudapati, V.; Monsel, A.; Park, J.H.; Hu, S.; Kato, H.; Lee, J.H.; Zhou, L.; He, H.; Lee, J.W. Mesenchymal Stem Cell–Derived Extracellular Vesicles Decrease Lung Injury in Mice. J. Immunol. 2019, 203, 1961–1972.
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221.
- Yao, M.; Cui, B.; Zhang, W.; Ma, W.; Zhao, G.; Xing, L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021, 264, 118658.
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286.
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L967–L977.
- Li, J.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76.
- Wang, J.; Huang, R.; Xu, Q.; Zheng, G.; Qiu, G.; Ge, M.; Shu, Q.; Xu, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit. Care Med. 2020, 48, e599–e610.
- Shen, K.; Wang, X.; Wang, Y.; Jia, Y.; Zhang, Y.; Wang, K.; Luo, L.; Cai, W.; Li, J.; Li, S.; et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023, 62, 102655.
- Pei, Z.; Cen, J.; Zhang, X.; Gong, C.; Sun, M.; Meng, W.; Mao, G.; Wan, J.; Hu, B.; He, X.; et al. MiR-146a-5p delivered by hucMSC extracellular vesicles modulates the inflammatory response to sulfur mustard-induced acute lung injury. Stem Cell Res. Ther. 2023, 14, 149.
- Liu, J.-S.; Du, J.; Cheng, X.; Zhang, X.-Z.; Li, Y.; Chen, X.-L. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J. Chin. Med. Assoc. 2019, 82, 895–901.
- Xiao, K.; He, W.; Guan, W.; Hou, F.; Yan, P.; Xu, J.; Zhou, T.; Liu, Y.; Xie, L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020, 11, 863.
- Yi, X.; Wei, X.; Lv, H.; An, Y.; Li, L.; Lu, P.; Yang, Y.; Zhang, Q.; Yi, H.; Chen, G. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp. Cell Res. 2019, 383, 111454.
- Liu, J.-H.; Li, C.; Cao, L.; Zhang, C.-H.; Zhang, Z.-H. Exosomal miR-132-3p from mesenchymal stem cells alleviated LPS-induced acute lung injury by repressing TRAF6. Autoimmunity 2021, 54, 493–503.
- Wu, X.; Liu, Z.; Hu, L.; Gu, W.; Zhu, L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res. 2018, 370, 13–23.
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Zingarelli, B.; Fan, H. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care 2019, 23, 44.
- Xu, J.; Xu, D.; Yu, Z.; Fu, Z.; Lv, Z.; Meng, L.; Zhao, X. Exosomal miR-150 partially attenuated acute lung injury by mediating microvascular endothelial cells and MAPK pathway. Biosci. Rep. 2022, 42, BSR20203363.
More
