Neutrophil extracellular traps (NETs) were originally discovered as a part of the innate immune response of the host to bacteria. They form a web-like structure that can immobilize microorganisms or exhibit direct antimicrobial properties, such as releasing reactive oxygen species (ROS). Resistance to cancer therapy is an important prognostic factor that influences the survival rates of patients. As neutrophil activation and recruitment are present in most solid tumors, it is important to establish if and how the presence of NETs in the tumor microenvironment (TME) might influence the outcome of cancer therapy. In the past, low levels of circulating neutrophils were associated with higher survival rates for patients who underwent different cancer treatments, which was initially considered coincidental.
- neutrophil extracellular traps (NETs)
- NETosis
- cancer therapy resistance
- epithelioma
1. NETs Can Provide Resistance to Chemotherapy
2. NETs Can Provide Resistance to Immunotherapy
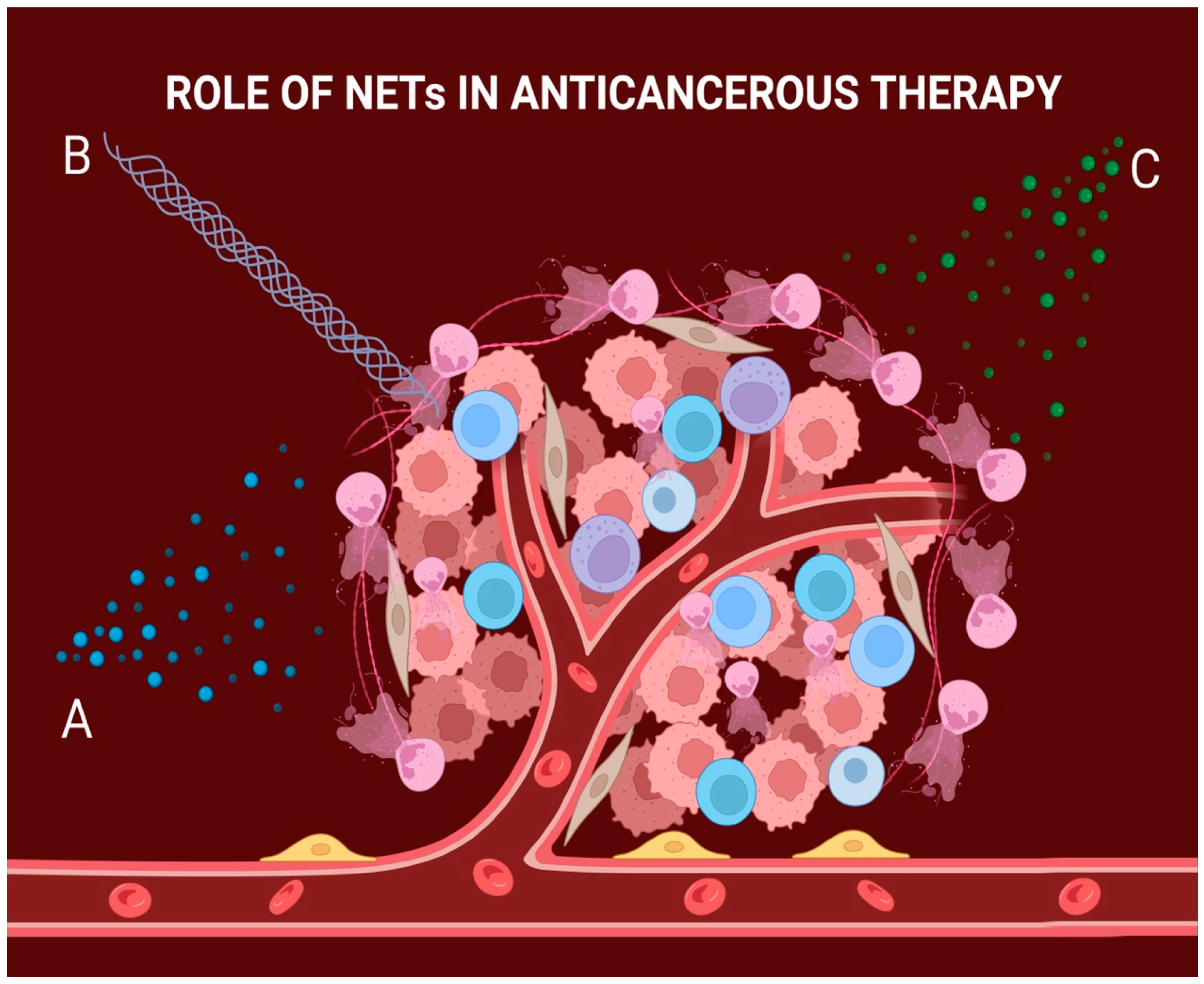
3. NETs Can Provide Resistance to Radiotherapy
| Role of NETs in Cancer | References |
|---|---|
| Regulate EndMT | [15,16,17][14][15][16] |
| Positive effect on tumor progression, invasion, and growth | [18,19,20,21][17][18][19][20] |
| Positive effect on angiogenesis | [22,23,24,25][21][22][23][24] |
| Provide resistance to chemotherapy | [26,27,28][25][26][27] |
| Provide resistance to immunotherapy | [31,32][28][29] |
| Provide resistance to radiotherapy | [33,,42][30]34,[31]35,[32]40[33][34] |
References
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503.
- Ramachandran, I.R.; Condamine, T.; Lin, C.; Herlihy, S.E.; Garfall, A.; Vogl, D.T.; Gabrilovich, D.I.; Nefedova, Y. Bone Marrow PMN-MDSCs and Neutrophils Are Functionally Similar in Protection of Multiple Myeloma from Chemotherapy. Cancer Lett. 2016, 371, 117–124.
- Lin, C.; Herlihy, S.E.; Li, M.; Deng, H.; Bernabei, L.; Gabrilovich, D.I.; Vogl, D.T.; Nefedova, Y. NETs Promote Tumor Resistance to Anthracyclines. Cancer Res. 2019, 79, 2103.
- Heinhuis, K.M.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.H.; Schellens, J.H.M. Enhancing Anti-Tumor Response by Combining Immune Checkpoint Inhibitors with Chemotherapy in Solid Tumors. Ann. Oncol. 2019, 30, 219–235.
- Zhang, Y.; Liu, G.; Sun, M.; Lu, X. Targeting and Exploitation of Tumor-Associated Neutrophils to Enhance Immunotherapy and Drug Delivery for Cancer Treatment. Cancer Biol. Med. 2020, 17, 32–43.
- Zhang, Y.; Chandra, V.; Sanchez, E.R.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-Induced Neutrophil Extracellular Traps Mediate Resistance to Checkpoint Blockade in Pancreatic Cancer. J. Exp. Med. 2020, 217, e20190354.
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for Optimizing the Response of Cancer and Normal Tissues to Radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542.
- Wisdom, A.J.; Hong, C.S.; Lin, A.J.; Xiang, Y.; Cooper, D.E.; Zhang, J.; Xu, E.S.; Kuo, H.C.; Mowery, Y.M.; Carpenter, D.J.; et al. Neutrophils Promote Tumor Resistance to Radiation Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 18584–18589.
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620.
- Shinde-Jadhav, S.; Mansure, J.J.; Rayes, R.F.; Marcq, G.; Ayoub, M.; Skowronski, R.; Kool, R.; Bourdeau, F.; Brimo, F.; Spicer, J.; et al. Role of Neutrophil Extracellular Traps in Radiation Resistance of Invasive Bladder Cancer. Nat. Commun. 2021, 12, 1–14.
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 Promotes Neutrophil Extracellular Trap Formation through Interactions with Toll-like Receptor 4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, 8–12.
- Kim, S.W.; Lee, H.; Lee, H.K.; Kim, I.D.; Lee, J.K. Neutrophil Extracellular Trap Induced by HMGB1 Exacerbates Damages in the Ischemic Brain. Acta Neuropathol. Commun. 2019, 7, 94.
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016, 8, 361ra138.
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil Extracellular Traps in Cancer: Not Only Catching Microbes. J. Exp. Clin. Cancer Res. 2021, 40, 231.
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty Acid Transport Protein 2 Reprograms Neutrophils in Cancer. Nature 2019, 569, 73–78.
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662.
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil Extracellular Traps: From Physiology to Pathology. Cardiovasc. Res. 2022, 118, 2737–2753.
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil Extracellular Traps in Breast Cancer and beyond: Current Perspectives on NET Stimuli, Thrombosis and Metastasis, and Clinical Utility for Diagnosis and Treatment. Breast Cancer Res. 2019, 21, 145.
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380.
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.U.; Varricchi, G.; Galdiero, M.R. Neutrophil Extracellular Traps in Cancer. Semin. Cancer Biol. 2022, 79, 91–104.
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (Nets) in Cancer Metastasis. Cancers 2021, 13, 6131.
- Söderberg, D.; Segelmark, M. Neutrophil Extracellular Traps in Vasculitis, Friend or Foe? Curr. Opin. Rheumatol. 2018, 30, 16–23.
- Kaltenmeier, C.; Yazdani, H.O.; Morder, K.; Geller, D.A.; Simmons, R.L.; Tohme, S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2021, 12, 785222.
- Li, P.; Lu, M.; Shi, J.; Hua, L.; Gong, Z.; Li, Q.; Shultz, L.D.; Ren, G. Dual Roles of Neutrophils in Metastatic Colonization Are Governed by the Host NK Cell Status. Nat. Commun. 2020, 11, 4387.
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773.
- Pieterse, E.; Rother, N.; Garsen, M.; Hofstra, J.M.; Satchell, S.C.; Hoffmann, M.; Loeven, M.A.; Knaapen, H.K.; Van Der Heijden, O.W.H.; Berden, J.H.M.; et al. Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1371–1379.
- Demers, M.; Wong, S.L.; Martinod, K.; Gallant, M.; Cabral, J.E.; Wang, Y.; Wagner, D.D. Priming of Neutrophils toward NETosis Promotes Tumor Growth. Oncoimmunology 2016, 5, e1134073.
- Miller-Ocuin, J.L.; Liang, X.; Boone, B.A.; Doerfler, W.R.; Singhi, A.D.; Tang, D.; Kang, R.; Lotze, M.T.; Zeh, H.J. DNA Released from Neutrophil Extracellular Traps (NETs) Activates Pancreatic Stellate Cells and Enhances Pancreatic Tumor Growth. Oncoimmunology 2019, 8, e1605822.
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased Neutrophil Extracellular Traps Promote Metastasis Potential of Hepatocellular Carcinoma via Provoking Tumorous Inflammatory Response. J. Hematol. Oncol. 2020, 13, 3.
- Milette, S.; Quail, D.F.; Spicer, J.D. Neutrophil DNA Webs Untangled. Cancer Cell 2020, 38, 164–166.
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138.
- Webb, N.J.A.; Myers, C.R.; Watson, C.J.; Bottomley, M.J.; Brenchley, P.E.C. Activated Human Neutrophils Express Vascular Endothelial Growth Factor (VEGF). Cytokine 1998, 10, 254–257.
- Scapini, P.; Calzetti, F.; Cassatella, M.A. On the Detection of Neutrophil-Derived Vascular Endothelial Growth Factor (VEGF). J. Immunol. Methods 1999, 232, 121–129.
