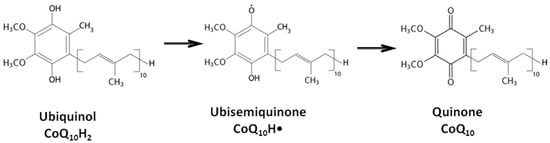
Coenzyme CoQ10 (CoQ10) is an endogenous lipid-soluble antioxidant that effectively protects lipids, proteins, and DNA from oxidation due to its ability to undergo redox transitions between oxidized and reduced forms. Various oxidative stress-associated infectious and somatic diseases have been observed to disrupt the balance of CoQ10 concentration in tissues. As a high molecular weight polar lipophilic compound, CoQ10 exhibits very limited oral bioavailability, which restrains its therapeutic potential. Nevertheless, numerous studies have confirmed the clinical efficacy of CoQ10 therapy through oral administration of high doses over extended time periods. Experimental studies have demonstrated that in emergency situations, intravenous administration of both oxidized and reduced-form CoQ10 leads to a rapid increase in its concentration in organ tissues, offering protection for organ tissues in ischemic conditions. This suggests that the cardio- and neuroprotective efficacy of intravenously administered CoQ10 forms could present new opportunities in treating acute ischemic conditions.
- coenzyme Q10
- ubiquinol
- ubiquinone
- brain ischemia
- myocardial infarct
- intravenous administration
1. Introduction
2. Biological Functions of Coenzyme Q10
3. Efficacy of Coenzyme Q10 Therapy When Administered Orally
The limited clinical efficacy of CoQ10 observed in several studies may be attributed to its low oral bioavailability and short duration of therapy, which result in low CoQ10 levels in both plasma and tissues and thus hinder CoQ10 from achieving its therapeutic potential. For instance, in a study involving patients experiencing clinical cardiac arrest, a seven-day course of CoQ10, despite a dosage of 300 mg taken twice daily resulting in increased plasma CoQ10 levels, failed to demonstrate an improvement in neurological and biochemical parameters
[58]
[59].
It should be noted that most clinical studies on the effectiveness of Coenzyme Q10 have been conducted using ubiquinone. There is not yet enough data to compare the effectiveness of ubiquinone and ubiquinol when taken orally due to the small number of clinical studies with ubiquinol
[60].
].4. Results of Intravenous Coenzyme Q10 Administration in Experimental Models In Vivo
In emergency situations like myocardial infarction or ischemic stroke, where acute CoQ10 deficiency is evident, alternative routes of administration, particularly intravenous (IV) delivery, may be more effective [61].
The major advantage of intravenous administration is CoQ10’s rapid penetration into tissues, which significantly increases CoQ10 tissue concentration and enables the achievement of substantial anti-ischemic potential during acute ischemia and reperfusion.
An acute oxygen deficiency is at the core of ischemic damage in any organ, which leads to disruption of the normal function of mitochondria, the main consumers of oxygen and ATP generators. CoQ10, being an integral component of the mitochondrial respiratory chain, facilitates electron transfer between complexes I and III, as well as II and III, and ensures normal mitochondrial function [62][63][62,63].
An increased CoQ10 tissue level in the form of ubiquinol, administered via intravenous injection, can effectively restrict the escalating electron leakage from the mitochondrial respiratory chain during ischemia and reduce the formation of harmful ROS, which damage the cell’s structural components. Notably, by administering coenzyme Q10 during ischemia, we safeguard mitochondrial function in the “risk zone” and prevent the progression of irreversible reactions leading to extensive cellular and tissue death.
Vasospasm, being a consequence of oxidative stress, exacerbates tissue damage [64][64]. In brain tissue, vasospasm accompanies “cortical spreading depolarization” (SD) [65]. Rapid vasodilation resulting from CoQ10’s intravenous administration impacts endothe-lial function, potentially reducing post-ischemic vasospasm and subsequent lesion vol-umes [12].
During ischemia coupled with tissue reperfusion, in addition to the aforementioned mechanisms of anti-ischemic protection, CoQ10’s antioxidant properties start playing a pivotal role [66].
Another pathogenetic mechanism of ischemic injury is inflammation associated with oxidative stress. CoQ10’s anti-inflammatory effects manifest at the onset of ischemia. These effects are attributed to CoQ10’s membrane-stabilizing and antioxidant actions, as well as CoQ10′ inhibition of inflammation-related cytokine gene expression and the ele-vation of anti-inflammatory biomarkers levels [33][67][33,67].
In addition to the previously mentioned mechanisms, CoQ10’s anti-ischemic action involves anti-apoptotic effects, increasing the levels of ubiquitin proteins and enhancing autophagy. Furthermore, CoQ10 decreases the levels of angiotensin-converting enzyme (ACE), preventing myocardial remodeling [68].
A meta-analysis of 6 published preclinical studies involving 116 animals with myo-cardial infarction modeling reperfusion confirmed the cardioprotective efficacy of CoQ10. On average, regardless of the model of myocardial ischemia or animal species used, CoQ10 administration resulted in a reduction of the infarct area by 11.36% compared to control groups [66].
However, research on the efficacy of CoQ10 through intravenous administration remains limited [69][69], primarily due to the lack of dosage forms approved for clinical use [70][71][72][70–72]. Animal studies have explored micellar or liposomal forms of CoQ10 [72] and solubilizers, such as HCO-60 (polyoxyethylene hydrogenated castor oil-60)[73] [73] or caspofungin [70]. Intracoronary administration of liposomal CoQ10 to rabbits at a dosage of 36 mg before a 30-min occlusion of the left coronary artery followed by 3 h of reperfu-sion resulted in a more than twofold reduction in the myocardial necrosis zone [74]. In rats, intramuscular administration of coenzyme Q10 at a dose of 20 mg/kg for 7 days prior to coronary artery occlusion led to a 57% reduction in the infarct zone area. This treatment normalized hemodynamic parameters and also lowered the levels of inflammatory and oxidative stress markers [75].
A novel CoQ10 dosage form designed for intravenous administration, based on solubilized ubiquinol, has successfully completed preclinical studies [76].
The pharmacokinetics of this new form of ubiquinol was studied in rats in compar-ison with ubiquinone, both forms having a similar excipient composition [77]. Both ki-netic curves were found to be biphasic, with a comparable initial decrease rate in plasma concentration during the first hours after injection. However, ubiquinone demonstrated significantly higher plasma CoQ10 levels between 24 and 96 h after administration and equalized with ubiquinol by the eighth day after administration. The area under the curve (AUC192 h) for ubiquinone was 1.5 times higher than that for ubiquinol. Accordingly, the total clearance of ubiquinol was 1.5 times higher than that of ubiquinone.
A single intravenous injection of CoQ10 at a dosage of 30 mg/kg leads to an imme-diate increase in its concentration in blood plasma by several times. This elevated con-centration persists for several hours, significantly exceeding the CoQ10 concentration in organ tissues. The transfer of CoQ10 from plasma to organs is accelerated, and a signifi-cant increase in CoQ10 concentration in tissues is observed as early as 15 min after ad-ministration. The rat tissue distribution of CoQ10 after injection showed similar patterns for both forms: in 15 min, its concentration increased by 2.5 times in the heart, by 1.7–2.0 times in the brain, and by 3.5 times in the kidneys. CoQ10 concentration remained ele-vated (by 70–50%) for at least 48 h upon injection. In the liver, CoQ10 accumulated gradually, peaking in 1–2 days, with maximum levels not significantly different between the forms, exceeding baseline levels by 17–23 times. Even on the eighth day, CoQ10 con-centration in the liver remained substantially (7–10 times) higher than baseline levels.
Comparative analysis of AUC and total body clearance (Clt) values between ubiq-uinone and ubiquinol revealed that ubiquinol was eliminated faster from blood plasma, and reached maximum concentration in the liver earlier. Notably, the total CoQ10 amount accumulating in the liver remained consistent regardless of administration in oxidized or reduced form. The accumulation and subsequent secretion of CoQ10 into the blood via lipoproteins might contribute to its sustained elevated concentration levels in plasma and tissues over time [77].
Following intravenous ubiquinol injection, CoQ10 redox status—defined as the proportion of the reduced form in the total pool—in blood plasma remained constant during the initial 48 h at the level of 92%. After the injection of the CoQ10 oxidized form in blood, a gradual reduction occurred, with the ubiquinol proportion reaching ap-proximately 89% by the end of the first day. This level is assumed to reflect the endoge-nous CoQ10 redox balance in rat blood plasma, analogous to human levels [25].
Notably, in the myocardium and brain, the proportion of the CoQ10 reduced form was significantly lower than in plasma, remaining constant throughout the entire obser-vation period: prior to ubiquinol administration, during the initial 96 h of elevated tissue concentration, and after returning to baseline levels by the eighth day. Thus, CoQ10 redox status is specific for each tissue of the organism and remains unchanged when CoQ10 tissue levels are increased as a result of intravenous administration. The revealed con-stancy of CoQ10 redox status, irrespective of the variations in absolute concentrations, suggests the presence of mechanisms governing CoQ10 redox status. Clear differences in CoQ10 redox status in blood plasma and organs indicate partial oxidation of ubiquinol to reach the level of endogenous redox balance upon the transfer from blood into organ tissues, involving the drug in local redox processes [25].
The rapid replenishment of CoQ10 tissue concentration and enhanced antioxidant capacity through intravenous administration, as revealed in pharmacokinetic studies, holds promise for acute ubiquinone deficiency, particularly in urgent ischemic conditions. The cardio- and neuroprotective efficacy of IV CoQ10 has beenaffirmed in experimental models of myocardial and brain infarction in animals. Pharmacokinetic research of intravenous administration at a dose of 30 mg/kg supports the use of such doses in experimental pathology models [78][79][80][81][82][83][84][78–74].
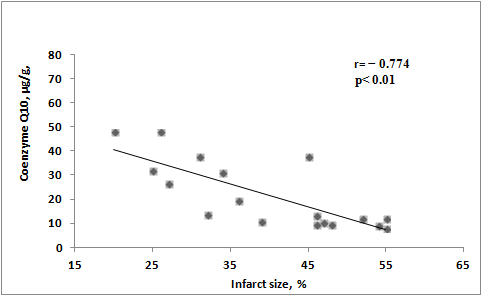
The cardioprotective effects of preventive ubiquinone administration has been demonstrated in a rat model of ischemia–reperfusion. Animals were intravenously in-jected with ubiquinone or saline 30 min before coronary artery occlusion. After 30 min of ischemia and 120 min of reperfusion, the area of left ventricular infarction and the level of CoQ10 in the myocardium were assessed. At reperfusion initiation, arrhythmias were observed in 8 out of 9 rats receiving saline, contrasting with only 2 out of 9 rats receiving ubiquinone. In the ubiquinone group, arrhythmias appeared later and were of shorter duration compared with untreated animals. Additionally, in the group of animals re-ceiving ubiquinone, the CoQ10 concentration was twice as highin the left ventricle, and the infarct area was one-third less than in the untreated group. Correlation analysis re-vealed that higher CoQ10 concentrations in myocardial tissue corresponded to smaller infarct sizes (Figure 2) [78].
![Figure 2. Correlation between CoQ10 levels in LV of infarct rats and LV infarct size [78].](/media/item_content/202402/figure2-65cccebfba734.jpg)
Figure 2.
Correlation between CoQ
10
levels in LV of infarct rats and LV infarct size [78].
Further investigations on the cardioprotective effects of ubiquinone administration in irreversible myocardial ischemia models revealed promising results. In rats, ubiquinone was administered intravenously 10 min after coronary artery occlusion. By day 21 after myocardial infarction, CoQ10 concentration in plasma, left ventricle, and liver in these animals was higher than in untreated rats by 87%, 23%, and 1042%, respectively. The size of the myocardial necrosis zone was smaller, and postinfarction hypertrophy was less severe in rats treated with CoQ10. These rats had higher values of stroke volume (by 24.6%), stroke work (by 34.9%), cardiac output (by 37.8%), ejection fraction (by 35.7%), contractility (by 22.5%), and lower end-diastolic pressure (by 25.8%) than untreated animals [79]. CoQ10 was shown to have cardioprotective efficacy when administered 60 min after occlusion [80].
The cardioprotective efficacy of intravenous ubiquinol administration was demonstrated in the same model of irreversible myocardial ischemia. Intravenous administration of ubiquinol (10 mg/kg) within 10 min after coronary artery occlusion resulted in a significant reduction of left ventricular myocardial aneurysm size on the 21st day (13.19% vs. 31.55% for treated and untreated groups, respectively). It also prevented the development of left ventricular myocardial hypertrophy and helped to control the decrease of cardiac pumping function. Additionally, in the treated animal group, an inverse correlation between CoQ10 concentration in the myocardium and interventricular septal thickness (r = −0.672, p< 0.05) was found, which emphasizes its role in controlling post-infarction damage. This result was comparable to the efficacy observed with intravenous administration of a higher dose (30 mg/kg) of oxidized CoQ10 [81].
Thus, a single intravenous injection of both oxidized and reduced forms of CoQ10 before or during myocardial ischemia elevates its concentration in the myocardium and exerts cardioprotective effects, minimizing the infarct zone, controlling myocardial hypertrophy, and enhancing functional heart characteristics.
Moreover, the neuroprotective CoQ10 effect has been demonstrated via intravenous administration in models of reversible and irreversible brain ischemia [82][83][84][82–84], and the correlations between CoQ10 tissue concentration and damage zone sizes have been established (Figure 3) [84].
![Figure 3 Correlation between area of the infarct region and CoQ10 tissue concentration in the ipsilateral hemisphere[84].](/media/item_content/202402/figure3-65cccea22cd82.jpg)
Figure 3. Correlation between area of the infarct region and CoQ10 tissue concentration in the ipsilateral hemisphere [84].
Correlation between area of the infarct region and CoQ10 tissue concentration in the ipsilateral hemisphere[84].
In male Wistar rats, reversible ischemia was induced viamiddle cerebral artery occlusion for 60 min followed by reperfusion. A single intravenous injection of ubiquinone or saline was administered 15 min before reperfusion. Sensory and motor functions, cerebral infarct volume, and CoQ10 concentration were assessed either 1 or 7 days later. Cerebral ischemia resulted in a significant decrease in endogenous CoQ10 concentration in both hemispheres. Intravenous ubiquinone injection increased its concentration in both hemispheres up to the concentration level in a sham group, and significantly improved neurological status and reduced the volume of cerebral infarction by 67% at day 1 and by 35% at day 7 following artery occlusion [82].
Similarly, in experimental models of irreversible cerebral ischemia resulting from middle cerebral artery occlusion, the neuroprotective efficacy of ubiquinone has also been demonstrated. Ischemic stroke was accompanied by a decrease in CoQ10 levels in both ipsilateral and contralateral hemispheres. Intravenous ubiquinone administration increased its concentration in both hemispheres. At 24 h, the neurological status of animals that received ubiquinone injection within 60 min after the onset of ischemia, as compared to untreated animals, was significantly better, mainly due to motor function improvement; also, the volume of brain necrosis was half as great [83][82]. Thus, it was shown that intravenous administration of ubiquinone in models of transient and chronic cerebral ischemia is accompanied by CoQ10 permeation into the brain and the achievementof neuroprotective effect.
A comparative study of neuroprotective efficacy between ubiquinone and ubiquinol in a reversible cerebral ischemia model during one day administering the drugs intravenously 15 min before reperfusion was carried out [84]. On the first day after the onset of ischemia, a decrease in mortality up to 10% compared to 57% in the control group, an improvement to neurological status, and brain necrosis abatement were revealed. At the same time, CoQ10 concentration in the brain tissue was found to correlate with both the size of the necrotic region and the neurological status of the animals (Figure 3Figures 3 and Figure 44).
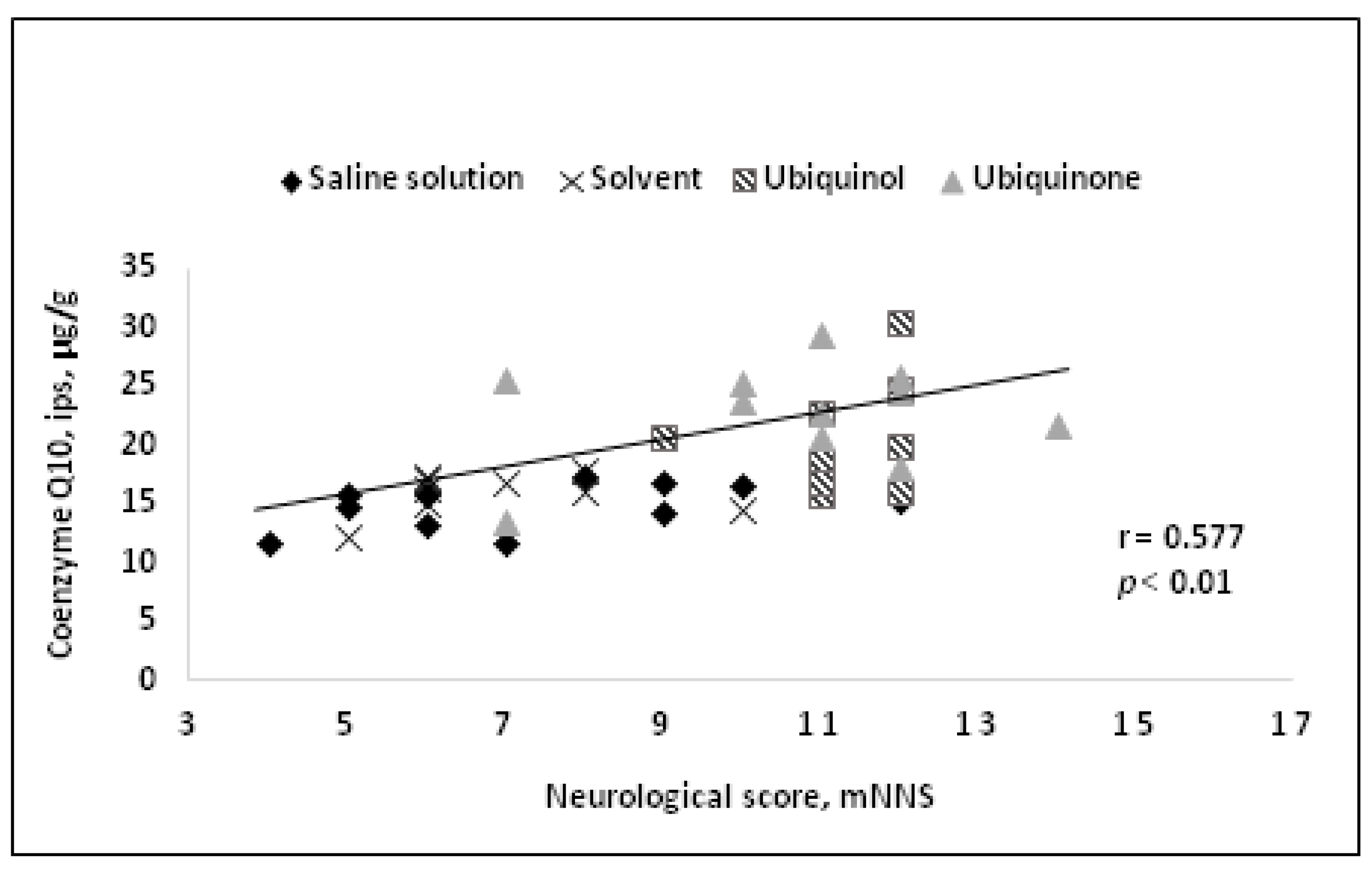
![Figure 4.Correlation between neurological deficit and CoQ10 tissue concentration in the ipsilateral hemisphere[84].](/media/item_content/202402/figure4-65cccef43ea3e.jpg) Figure 4.
Figure 4.
Correlation between neurological deficit and CoQ10 tissue concentration in the ipsilateral hemisphere [84].
Correlation between neurological deficit and CoQ10 tissue concentration in the ipsilateral hemisphere[84].
A significant decrease in CoQ10 tissue concentration after ischemia in untreated animals in both ipsilateral and contralateral hemispheres was noted as early as by the end of the first day, followed by further CoQ10 concentration decrease by the end of the fourth day. Intravenous administration of both ubiquinol and ubiquinone in treated animals helped to increase CoQ10 concentration levelswithin 24 h compared with a sham group. By the day 4 after ubiquinol injection, the CoQ10 concentration in both hemispheres in animals remained at the level observed in the sham group.
In the same study, the neuroprotective efficacy of CoQ10 at 4 days was evaluated using ubiquinol as an example. The mortality of animals in the control group on day 4 reached 80%, compared to 20% mortality rate in the group treated with ubiquinol. The neurological deficit in ubiquinol-administered animals did not worsen, unlike in the untreated group. MRI assessment was used to evaluate changes in the size of the lesion in each animal during 4 days. Within this period, there was an almost twofold increase in the area of the brain lesion in the control group. In the group of animals treated with ubiquinol injection, the size of necrotic region did not increase compared to the size of necrotic region by the end of the first day.
Using the same model of focal rat brain ischemia and an intravenous route of CoQ10 administration at the same dose of 30 mg/kg, the following was demonstrated: improved neurological status of treated animals in terms of sensory–motor functions, decreased necrosis, enhanced viability of blood–brain barrier, and reduced brain edema. All of the above findings are consistent with ouresearchers' results. The neuroprotective efficacy of CoQ10 is attributed to the reduction of proinflammatory cytokines and an involvement in molecular mechanisms related to miR-149-5p gene expression [67].
Thus, a single intravenous injection of either oxidized or reduced forms of CoQ10 in experimental cerebral ischemia increases the CoQ10 concentration in both cerebral hemispheres. This manifests considerable neuroprotective effects, including mitigating the necrotic region, preventing its expansion, and preserving neurological status. The observed inverse correlation between the lesion size and CoQ10 concentration in tissues further supports the neuroprotective potential of CoQ10.
These experimental findings underscore the potential of intravenous CoQ10 administration to offer substantial cardio- and neuroprotective effects in cases of heart and brain ischemia, irrespective of CoQ10 redox state.
5. Conclusions
The administration of both oxidized and reduced