In the diverse arsenal of therapeutic tools against cancer, immune checkpoint inhibitors (ICI) have emerged as a new beacon of hope. By inhibiting the immune response’s “OFF” signal, ICIs activate the body’s immune system to attack cancerous growths. Eight immune checkpoint inhibitors have been approved by the FDA for their proven efficacy against multiple cancer types. Per their mechanism of action, ICIs produce a series of well-documented side effects secondary to the induction of immune activation commonly referred to as “immune-related adverse events” (IRAEs). These can affect any organ system, including the eye. Although rare, ocular IRAEs can have debilitating effects on patients’ quality of life and be sight-threatening.
1. Brief Overview of Immune Checkpoint Inhibitors
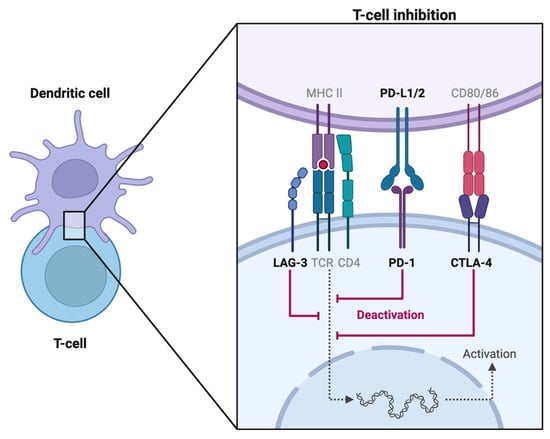
1.1. CTLA-4 Inhibitors
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a transmembrane receptor preferentially expressed on regulatory T cells (Treg) and memory T cells
[1]. Acting as a homodimer, it antagonizes CD28 signaling and suppresses the T cell response by competitively binding to CD80 and CD86, proteins expressed by antigen-presenting cells (APCs), as per
Figure 1. Through a complex signal transduction pathway, CTLA-4 activation leads to reduced IL-2 secretion which limits T cell expansion and differentiation
[2]. The CTLA-4 neutralizing antibody ipilimumab (Yervoy) was the first ICI approved by the FDA in 2011.
1.2. PD-1 Inhibitors
Programmed Cell Death Protein 1 (PD-1) is a cell surface protein expressed on natural killer (NK) cells, APCs (B cells, macrophages, dendritic cells), and activated T cells. Once a T-cell receptor (TCR)/CD28 interaction forms, PD-1 can be expressed, binds to programmed death ligand-1 (PD-L1) ligand, and mediates dephosphorylation of the TCR via the phosphatidylinositol 3-kinase (PI3K) pathway
[3]. Thereby, PD-1/PD-L1 signaling antagonizes the positive feedback loop induced by TCR/CD28 binding, which ultimately halts cell cycle progression on both innate and adaptive immune responses
[4]. In murine models lacking PD-1, a higher likelihood of developing autoimmune diseases has been observed
[5][6][5,6].
1.3. PD-L1 Inhibitors
PD-L1 is found on target tissues and binds to PD-1. Via a complex signaling pathway involving ZAP70 phosphorylation, PD-1/PD-L1 interaction inhibits T cell proliferation in the lymph node. PD-L1 expression is upregulated in various malignancies, particularly lung cancers. On the other hand, in autoimmune diseases like systemic lupus erythematosus, APCs fail to express PD-L1
[7].
1.4. LAG-3 Inhibitor
Lymphocyte activation gene-3 (LAG-3) found on activated T cells, inhibits T cell mitochondrial activity and is associated with CD4/CD8+ T cell exhaustion, making it a promising target for novel ICI therapies
[8][9][10][8,9,10]. Studies have found that inhibiting LAG-3 and PD-1 led to increased cytotoxic T-cell activity and tumor response
[11][12][11,12]. In March 2022, the FDA approved the first LAG-3 inhibitor, relatlimab, for use against unresectable or metastatic melanoma. The administration dose is 480 mg nivolumab plus 160 mg relatlimab intravenously over 30 min every 4 weeks
[13]. No cases of relatlimab-linked uveitis have been reported as of the writing of this
resear
chticle.
2. Side Effects Other Than Ocular Side Effects
The incidence of IRAEs ranges between 64 and 72%, with high-grade IRAEs affecting up to 18–24% of patients undergoing ICI
[14]. The timeline for the occurrence of IRAEs varies greatly. Though most IRAEs appear within 3–6 months of ICI initiation, delayed responses may take up to a year to appear, posing a challenge to diagnosis
[15][16][17][15,16,17]. The incidence of IRAEs increases in a dose-dependent manner
[14][18][14,18]. In a systematic review comparing ipilimumab 3 mg/kg and 10 mg/kg, authors found that the higher dosage group had a 3.10 greater chance of developing high-grade IRAEs
[14]. This is not necessarily undesirable, however. In Downey et al. (2007), among 139 patients treated with ipilimumab for metastatic melanoma, all patients who experienced complete responses developed severe IRAEs, and the relationship between IRAEs and response was statistically significant
[19]. The presence of IRAEs is positively correlated with increased survival. In a retrospective study of 133 patients, the overall survival of patients who developed IRAEs was thrice that of those who did not (37.8 months versus 10.1 months, respectively). The same study also found that patients who discontinued ICI had a mean survival time 30% shorter than those whose therapy was uninterrupted despite IRAEs
[20]. Though a higher disease severity may be a confounding factor between increased mortality and ICI interruption, the clinical reflex to cease cancer therapy at once as side effects appear may need reconsideration. The non-ocular side effects of ICIs extend into a range of organ systems as illustrated in
Table 1.
Table 1.
Non-ocular adverse events associated with ICI therapy.
Note: common side effects (those affecting between 1 in 10 and 1 in 100 people) are in bold [14][20][14,20].
3. Ocular and Orbital Side Effects Other Than Uveitis
Ocular side effects associated with ICI therapy are generally rare and occur alongside other systemic IRAEs. Though their combined incidence is only around 1%–2.8%, early detection is necessary to prevent significant impacts on patient’s vision and quality of life
[44][45][44,45]. Among phase I-III trials, dry eyes were the most reported IRAEs, with an incidence ranging from 1.2 to 24.2%, followed by uveitis at 0.3% to 6%. There were no reported cases of high-grade dry eyes, and only one small phase I study for combined nivolumab and ipilimumab in advanced melanoma reported more than one case of high-grade uveitis (two cases, or 4%)
[44][46][44,46]. Most ocular IRAEs were reported in patients undergoing treatment for advanced or metastatic melanoma
[44]. Though a wide range of ocular IRAE manifestations can be found in published case reports, as illustrated further in this section, interestingly, few diagnostic details are provided in larger drug trial studies. One study characteristically grouped all ocular symptoms under “dry eyes/blurred vision”
[47]. Therefore, a close collaboration between ophthalmologists and oncologists is needed to better identify, prevent, and treat morbidity secondary to ICI while ensuring optimal continuation of ICI therapy.
Other than uveitis, some common ocular IRAEs (oIRAE) associated with the ocular system include:
- -
-
Orbit;
-
- ○
-
Giant cell arteritis
[48];
-
- ○
-
Myasthenia gravis
[49][50][49,50];
-
- ○
-
Inflammatory orbitopathy
[51];
-
- ○
-
Cranial nerve 3/6/7 palsy
[21][38][21,38];
-
-
- -
-
Anterior segment;
-
- ○
-
Dry eye [52][53][52,53];
-
- ○
-
Corneal ulcer
[51][54][51,54];
-
-
- -
-
Posterior segment
-
- ○
-
Choroidal neovascular membrane/choroidal effusion
[42][55][56][42,55,56];
-
Note: common side effects (those affecting between 1 in 10 and 1 in 100 people) are
in bold [14][20][14,20].