Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Evangelia Argyropoulou and Version 2 by Mona Zou.
The COVID-19 pandemic has presented numerous challenges to the global healthcare system and emerging evidence suggests a potential link between COVID-19 treatment, specifically steroid therapy, and the development of avascular necrosis (AVN) of the hip. Understanding the nuances of AVN in the context of COVID-19 is crucial for healthcare professionals to navigate treatment decisions and mitigate potential complications.
- COVID-19
- AVN
- bone avascular necrosis
- osteonecrosis
- hip pain
- steroids
1. Introduction
The COVID-19 pandemic has presented numerous challenges to the global healthcare system, and as healthcare professionals continue to explore the long-term consequences of the infection, emerging evidence suggests a potential link between COVID-19 infection and treatment, specifically steroid therapy, and the development of avascular necrosis (AVN) of the hip.
The incidence of hip AVN in patients recovering from COVID-19 and receiving steroid therapy varies among studies and there is no report until now establishing the incidence of femoral head osteonecrosis (FHOn). Even though the exact cumulative steroid dose and the duration of COVID-19 treatment that can lead to AVN are not yet established, it is known that a longer treatment duration, the severity of infection, admission in the ICU, and the personal history count as potential risk factors [1][8]. Close follow up with MRI of the major joints is suggested for the first 3 months after a SARS infection, in order to treat the damage and save the joint, especially in young active patients (Figure 1). Symptoms of hip AVN typically manifested several weeks to months after COVID-19 infection and steroid treatment. The literature illustrates the importance of MRI in the diagnosis of AVN, and more specifically as an early-stage diagnosis [2][9], as it may aid in choosing between a surgical or nonsurgical type of treatment [3][10]. The femoral head is the most common location for ONC and is quite often bilateral [4][11], but signs of necrosis can be found in other parts like the shoulder, knee, and ankle [5][12]. There appears to be a correlation between the use of steroids, particularly methylprednisolone and dexamethasone, in the treatment of moderate to severe COVID-19 and the subsequent development of hip AVN [6][13]. Further research with larger cohorts and long-term follow ups is needed, to better understand the causative relationship and optimal management strategies for hip AVN in the context of COVID-19 and steroid therapy.
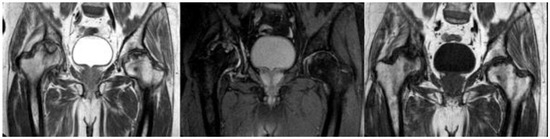
Figure 1. MRI presenting bilateral avascular necrosis of the hip after COVID-19 infection and treatment in ICU unit of our Hospital.
2. Demographics
Twenty-three articles are included in the study and there are 613 patients in total. In total, 62% of them are males and the rest are females. The estimated mean age is 42.9 years old, ranging from 19 to 63 years old. It is important to note that a lot of patients had bilateral joint pain and signs of AVN after the COVID-19 infection (Figure 1). The majority of the articles are case reports, illustrating mostly 1 patient and some of them reference up to 5 different patients [7][14]. The prospective study by Veizi et al. [2][9] is the biggest study so far, concerning the number of patients, with 472 people. All of the patients showed symptoms of hip osteonecrosis, except for the study by Kashkosh et al. [8][15], in which the patient had humeral head pain, only two days after the second dose of the Pfizer COVID-19 vaccine (Figure 23 and Figure 34).
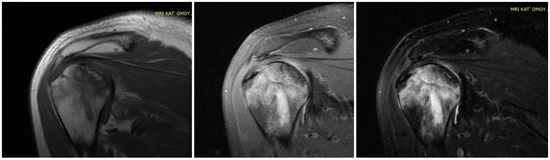
Figure 23. MRI presenting AVN of LEFT humerus head in a case of bilateral osteonecrosis after COVID-19 vaccination and infection treated in ICU with high dose of corticosteroids in our Hospital.
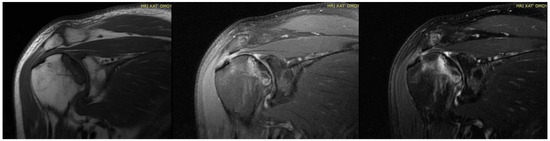
Figure 34. MRI presenting AVN of RIGHT humerus head (previous case of Figure 34) of bilateral osteonecrosis after COVID-19 vaccination and infection treated in ICU with high dose of corticosteroids in our Hospital.
3. Course of COVID-19 Infection
The infection’s severity varied, with mild, moderate, and severe cases being observed. Mild infections in the studies were scarce with only 11 patients, while the majority of the patients had mostly moderate and some moderate to severe symptoms. In total, 18 out of 23 articles are related to severe COVID-19 infection.
Clinical signs of musculoskeletal symptoms typically manifested within a range of days to a few months after the COVID-19 infection, with an average of 85 days. The shortest period is 14 days [9][16] and the longer duration for the AVN symptoms to appear is 300 days [10][17]. Generally, joint discomfort occurred after the resolution of acute respiratory symptoms and elevated body temperature.
4. COVID-19 Treatment
The treatment for moderate to severe cases consists of antiviral therapy and corticosteroids, which could be administered either intravenously or orally. Three studies combined the antiviral medication with steroids [11][12][13][18,19,20], while at the same time, three other studies did not use any corticosteroid [14][15][16][21,22,23]. However, Veizi et al. [2][9] divided the patients into two groups and half of them were treated with steroids and the rest without. Corticosteroids commonly used in COVID-19 treatment are dexamethasone, methylprednisolone, and prednisolone and are associated with potential complications such as femoral head osteonecrosis (FHOn). Their doses varied widely, ranging from 40 mg to 20,675 mg of prednisolone equivalents, while the treatment duration differed from a few days to several weeks. A cumulative dose of 2000 mg of prednisolone or an equivalent is linked to an increased risk for AVN. While corticosteroids have shown benefits in treating severe COVID-19 cases, the text raises concerns about potential long-term complications. It emphasizes the need for a balance between the benefits of treatment and the risks of complications.
5. AVN Treatment Approaches and Outcome Measures
In six studies, the patients had initially undergone conservative treatment, which was constituted by NSAIDS, physical therapy [11][15][18,22], intra-articular (IA) steroid injections, oral or IV steroid medication, and bisphosphonates [14][17][21,24]. Concerning the mobility, patients modified their activities and had protected bare weight. In one study, the patients received an IA hydrodilatation injection [8][15]. Out of those articles, the conservative treatment succeeded only in two studies, with clinical improvement [11][18] and a mean VAS score of 2.7 [17][24], while in the rest, the patients had surgical intervention, like core decompression, total hip arthroplasty (THA), and bone marrow aspirate concentrate (BMAC) injection. As for core decompression, it was combined with BMAC in three studies [12][18][19][19,25,26] out of the ten that it was used in. Furthermore, total hip arthroplasty was paired with the decompression of the necrosis foci in the study by Annam et al. [13][20] for a younger patient. For the rest of the patients, they were treated with THA, and the results indicated an improvement in motor activity and decrease in pain intensity, without a significant improvement in MRI though (Figure 45). The mean VAS score lessened from 9.4 to 2.8 in the first postoperative week [7][14]. Some cases did not receive specific treatment for AVN and in general the response to treatment varied, while some patients improved with conservative measures, while others required surgical intervention. Only one case involved infection post-THA and needed a two-stage THA [7][14]. As for the radiological outcomes, in some cases, there was improvement seen in imaging studies, but not in all. However, Veizi et al. [2][9] highlighted that there is no relationship between the treatment duration, cumulative dosage of medication, and ONC.
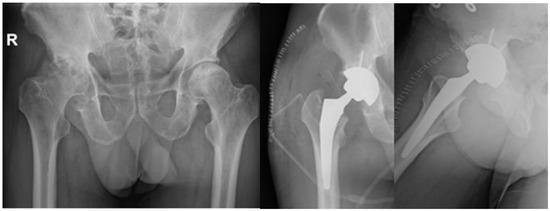
Figure 45. Final treatment of AVN after COVID-19 infection with total hip arthroplasty in our Department.
6. Follow-Up Period
The text highlights persistent symptoms, including fatigue, shortness of breath, anxiety, depression, joint pain, and stiffness, in COVID-19 survivors at a one-year follow up. Joint pain and stiffness are underreported in long-term studies. The majority of the studies had followed up with their patients for an average of 3–4 months and only one study had long-term outcomes at 25 months [20][27]. The impact of SARS-CoV-2 infection and therapeutic interventions on the skeletal system, especially AVN, is not thoroughly investigated. The text emphasizes the need for further studies to identify risk factors, determine the incidence of FHOn, and establish protocols for an early diagnosis and intervention. The study also highlights gaps in existing studies, such as inconsistent reporting of musculoskeletal symptoms and a lack of emphasis on FHOn incidence. It suggests that future research should include comprehensive musculoskeletal evaluations in the post-acute phase of COVID-19.
Table 1. Characteristics of the included studies.
| Scheme | Study Design | No. of Patients and Gender | Mean Age (years) | COVID-19 Symptoms | Treatment of COVID-19 | Days Until AVN | AVN Treatment | Results |
|---|---|---|---|---|---|---|---|---|
| 1. Sulewski et al. (2021) [21][5] | Cohort study | 10 (6 F + 4 M) | 58.8 | Moderately severe | Steroids | Initially conservatively (NSAIDs, IA steroid inj) with no improvement Oral dexamethasone at 2 × 8 mg daily for 2 weeks |
30% THA with good clinical outcome 10% chronic pain without joint destruction in the control tests |
|
| 2. Alkindi et al. (2021) [11][18] | Case report | 1 M | 29 | Combination of experimental anti-COVID-19 therapies (favipiravir, hydroxychloroquine, tocilizumab) Methylprednisolone IV at 40 mg/d for 5 days |
IV corticosteroids for 5 days (cumulative dose: 200 mg) |
Clinical improvement with NSAIDs and PT | ||
| 3. Daltro et al. (2021) [22][28] | Follow up | 14 M + 9 F | 43.5 | 33% Mild 66% Moderate/Severe |
Mild infection: no hospitalization/corticosteroids Dexamethasone: min dose of 40 mg/day for a mean time of 14.6 days (min 15–max 21) |
33% osteonecrosis of the femoral head | ||
| 4. Chacko et al. (2021) [23][29] | Case report | 1 M (bilateral) | 23 | IV dexamethasone at 6 mg/d for 9 days and IV methylprednisolone at 40 mg × 2/d for 5 days Cumulative dose is equivalent to 860 mg of prednisolone |
56 | Core decompression of femoral heads Bone marrow aspirate concentrate injection |
||
| 5. Joshi et al. (2021) [24][30] | Case report | 1 F (bilateral) | 31 | Moderate/Severe | Methylprednisolone at 32 mg/d for 7 days with minimal improvement Continued for 10 days more Total dose of 544 mg |
30 | N/G | |
| 6. Agarwala et al. (2021) [17][24] | Case report | 3 M (bilateral) | 37 | Moderate/Severe | Mean equivalent to 758 mg of prednisolone | 58 | Oral alendronate at 70 mg/w IV zoledronic acid at 5 mg annually |
Mean VAS: 2.7 No surgery |
| 7. Panin et al. (2022) [12][19] | Case report | 4 (2 M/2 F) (bilateral) | 34 | Moderate/Severe | Mean total dose of dexamethasone/prednisolone of 264 (80–600 mg)/1759 (533–4000 mg) 1 patient: iv favipiravir, tocilizumab 2nd: iv triazaverin |
96.6 | 2 decompressions of the necrosis foci Administration of a bone marrow concentrate 1THA Statins Bisphosphonates Anticoagulants |
Improvement in motor activity Decrease in pain intensity No significant improvement in MRI |
| 8. Uyshal et al. (2022) [14][21] | Case report | 1 M | 63 | Mild/Moderate | No steroid usage | Protected weight bearing Oral alendronate at 70 mg/w (no improvement) THA |
||
| 9. Ergün et al. (2022) [15][22] | Case report | 1 F (bilateral) | 51 | No prior use of steroid Favipiravir LMWH |
60 | Core decompression PT No weight bearing for 6 weeks |
Improved clinical scores No femoral head subchondral bone collapse |
|
| 10. Kingma et al. (2022) [10][17] | Case report | 1 M (bilateral) | 60 | Severe | Total dose of prednisone equivalent of 1327.5 mg | 300 | Bilateral THA | No complications |
| 11. Ardakani et al. (2022) [7][14] | Case series | 5 (2 M/3 F) | 38.4 | Moderate/Severe | Mean dose of prednisolone was 1695.2 mg | 41.6 | All patients underwent surgery with direct anterior approach 1 did two-stage THA due to Serratia marcescens infection in both hips |
Clinical and laboratory symptoms improved significantly Mean VAS decreased from 9.4 to 2.8 1 week post-operation |
| 12. Annam et al. (2022) [13][20] | Case report | 2 M (bilateral) | 48 (27/69) | Moderate | Oseltamivir, doxycycline, and methylprednisolone Mean total dose of methylprednisolone of 588 mg, equivalent to 735 mg of prednisolone |
The younger had bilateral THA and hip core decompression The older had left THA and right hip decompression |
||
| 13. Kamani et al. (2022) [25][31] | Case report | 1 M (bilateral) | 40 | Severe | Steroid injection | Bilateral core decompression hip surgery PT |
||
| 14. Kashkosh et al. (2022) [8][15] | Case report | 1 M | 40 | Second dose of the Pfizer COVID-19 vaccine | AVN of the humeral head | 2 | Analgesics Activity modification IA hydrodilatation inj |
Improved ROM Severe shoulder pain Surgical intervention |
| 15. Jyothiprasanth et al. (2023) [16][23] | Cohort study | 17 (10 M/7 F)/4 bilateral | 37 | 82.4% COVID-19 Inf | No steroid therapy | 66 | N/G | |
| 16. Baimukhamedov (2023) [3][10] | Cohort study | 8 M | N/G | N/G | Range of cumulative corticosteroid doses 50–20,675 mg of prednisolone |
N/G | N/G | |
| 17. Karpur et al. (2023) [26][32] | Follow up | 20 (14 M/6 F) | N/G | N/G | N/G | N/G | N/G | Stage I: 45% Stage II: 40% M/F: 70/30% |
| 18. Shershah et al. (2023) [18][25] | Case reports | 3 (2 M/1 F) bilateral | 29.3 | Severe | 560 mg of IV methylprednisolone | 240 | Conservative without results 2 bilateral THAs 1 core decompression with bone marrow aspirate infiltration |
|
| 19. Velchov et al. (2023) [20][27] | Follow up | 24 (17 M/7 F) 4 bilateral |
55.6 | 8 Moderate/16 Severe | Moderate: mean of 120 mg of dexamethasone Severe: also 3600 mg of methylprednisolone |
56.3 | 23 THAs 5 core decompressions |
|
| 20. Jayapalan et al. (2023) [19][26] | Case report | 1 M (bilateral) | 31 | Moderate | IV methylprednisolone (600 mg) followed by an oral dose of 8 mg | 65 | Bilateral core decompression and BMAC | |
| 21. Parikh et al. (2023) [27][33] | Case reports | 3 (2 M/1 F) | 55.6 | Moderate | Steroid treatment | N/G | Core decompression (1 bilateral) | |
| 22. Sinha et al. (2023) [9][16] | Cohort study | 10 (4 M/6 F) | 53.9 | 7 Moderate/3 Severe | 4 steroid therapies | 14 | 4 core decompressions | |
| 23. Veizi et al. (2023) [2][9] | Prospective study | 472 (289 M/183 F) | 42 | Group 2: (236) received steroid treatment | Increased % of AVN in Group 2 Joint pain: 5.1% in Group 1 11.9% in Group 2 AVN: 8 pts from Group 2 |
No relationship between the treatment duration, cumulative dosage of medication, and ONC |
N/G: Not Given.
