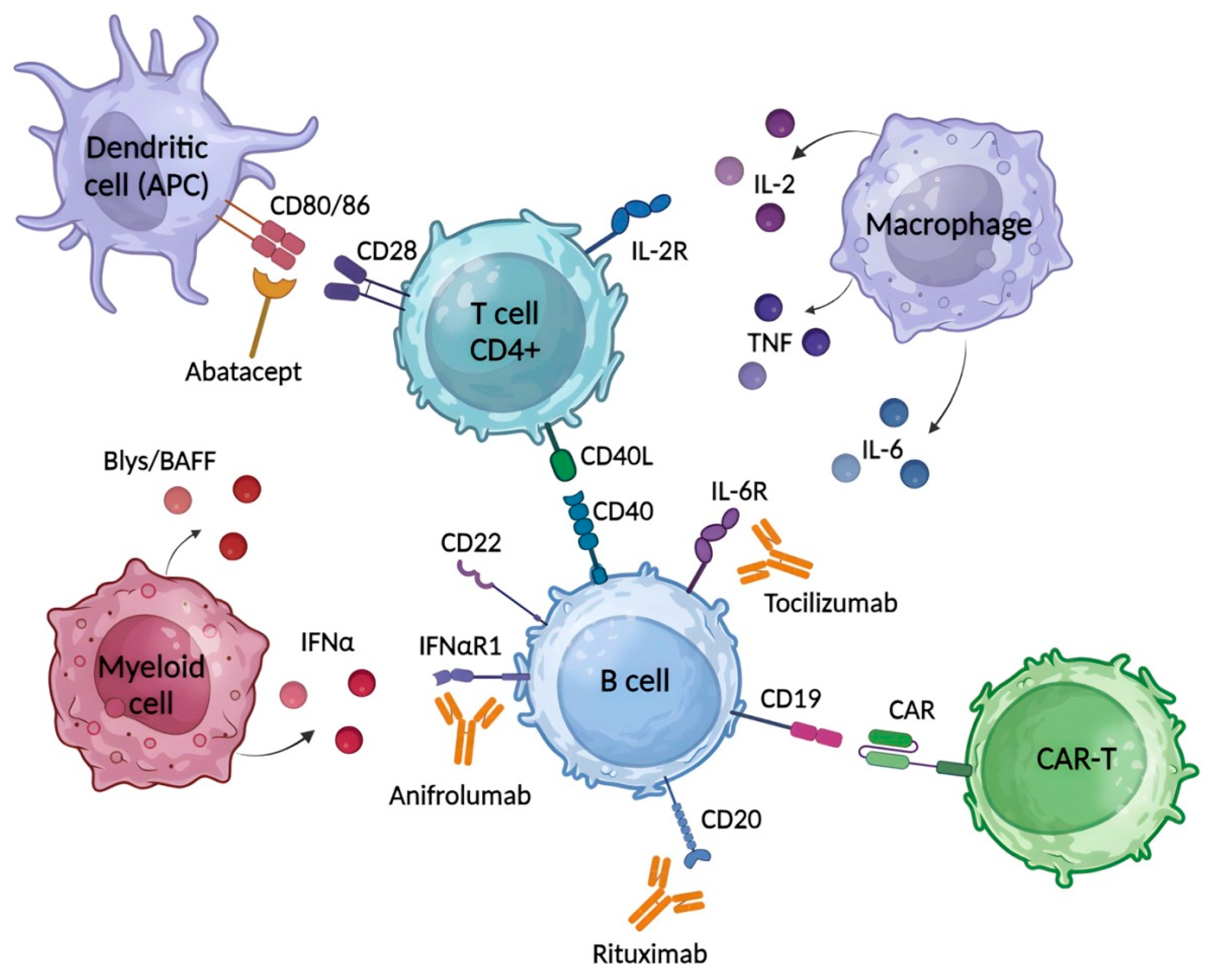
Systemic autoimmune diseases (SAIDs), such as systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and rheumatoid arthritis (RA), are fully related to the unregulated innate and adaptive immune systems involved in their pathogenesis. They have similar pathogenic characteristics, including the interferon signature, loss of tolerance to self-nuclear antigens, and enhanced tissue damage like necrosis and fibrosis. Glucocorticoids and immunosuppressants, which have limited specificity and are prone to tolerance, are used as the first-line therapy. A plethora of novel immunotherapies have been developed, including monoclonal and bispecific antibodies, and other biological agents to target cellular and soluble factors involved in disease pathogenesis, such as B cells, co-stimulatory molecules, cytokines or their receptors, and signaling molecules. Many of these have shown encouraging results in clinical trials. CAR-T cell therapy is considered the most promising technique for curing autoimmune diseases, with recent successes in the treatment of SLE and SSc.
- immunotherapy
- autoimmune diseases
- CAR-T cells
- systemic lupus erythematosus (SLE)
- systemic sclerosis (SSc)
- rheumatoid arthritis (RA)
1. Introduction
2. Common Therapeutics for SLE, SSc and RA
References
- Wang, L.; Wang, F.; Gershwin, M.E. Human Autoimmune Diseases: A Comprehensive Update. J. Intern. Med. 2015, 278, 369–395.
- Dima, A.; Jurcut, C.; Arnaud, L. Hydroxychloroquine in Systemic and Autoimmune Diseases: Where Are We Now? Jt. Bone Spine 2021, 88, 105143.
- Emamikia, S.; Gentline, C.; Chatzidionysiou, K.; Arnaud, L.; van Vollenhoven, R. Relationship between Glucocorticoid Dose and Adverse Events in Systemic Lupus Erythematosus: Data from a Randomized Clinical Trial. Scand. J. Rheumatol. 2018, 47, 131–140.
- Dubey, A.K.; Handu, S.S.; Dubey, S.; Sharma, P.; Sharma, K.K. Belimumab: First Targeted Biological Treatment for Systemic Lupus Erythematosus. J. Pharmacol. Pharmacother. 2011, 2, 317–319.
- Felten, R.; Dervovic, E.; Chasset, F.; Gottenberg, J.-E.; Sibilia, J.; Scher, F.; Arnaud, L. The 2018 Pipeline of Targeted Therapies under Clinical Development for Systemic Lupus Erythematosus: A Systematic Review of Trials. Autoimmun. Rev. 2018, 17, 781–790.
- Hafeez, U.; Gan, H.K.; Scott, A.M. Monoclonal Antibodies as Immunomodulatory Therapy against Cancer and Autoimmune Diseases. Curr. Opin. Pharmacol. 2018, 41, 114–121.
- Zhao, Q. Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date. BioDrugs 2020, 34, 111–119.
- Kalayci, F.N.C.; Ozen, S. Possible Role of Dysbiosis of the Gut Microbiome in SLE. Curr. Rheumatol. Rep. 2023, 25, 247–258.
- Pan, Q.; Guo, F.; Huang, Y.; Li, A.; Chen, S.; Chen, J.; Liu, H.; Pan, Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021, 12, 799788.
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022, 28, 2124–2132.
- Bergmann, C.; Müller, F.; Jörg, D.; Györfi, D.M.H.; Völkl, S.; Aigner, M.; Harrer, T.; Bayerl, N.; Atzinger, A.; Taubmann, J.; et al. AB0816 Treatment of a Patient with Severe Diffuse Systemic Sclerosis (SSC) Using CD19-Targeting CAR-T-Cells. In Scientific Abstracts; BMJ Publishing Group Ltd.: London, UK; European League Against Rheumatism: Zürich, Switzerland, 2023; p. 1621.
- Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Kudriaeva, A.; Miftakhova, R.; Rybalov, A.; Bulatov, E. Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies. Bioengineering 2022, 9, 808.
- Kaegi, C.; Wuest, B.; Schreiner, J.; Steiner, U.C.; Vultaggio, A.; Matucci, A.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Rituximab in Treating Immune-Mediated Disorders. Front. Immunol. 2019, 10, 1990.
- Edwards, J.C.W.; Szczepański, L.; Szechiński, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-Cell–Targeted Therapy with Rituximab in Patients with Rheumatoid Arthritis. N. Engl. J. Med. 2004, 350, 2572–2581.
- Merrill, J.T.; Neuwelt, C.M.; Wallace, D.J.; Shanahan, J.C.; Latinis, K.M.; Oates, J.C.; Utset, T.O.; Gordon, C.; Isenberg, D.A.; Hsieh, H.-J.; et al. Efficacy and Safety of Rituximab in Moderately-to-Severely Active Systemic Lupus Erythematosus: The Randomized, Double-Blind, Phase Ii/Iii Systemic Lupus Erythematosus Evaluation of Rituximab Trial. Arthritis Rheum. 2010, 62, 222–233.
- Kamburova, E.G.; Koenen, H.J.P.M.; Borgman, K.J.E.; ten Berge, I.J.; Joosten, I.; Hilbrands, L.B. A Single Dose of Rituximab Does Not Deplete B Cells in Secondary Lymphoid Organs but Alters Phenotype and Function. Am. J. Transplant. 2013, 13, 1503–1511.
- Maher, T.M.; Tudor, V.A.; Saunders, P.; Gibbons, M.A.; Fletcher, S.V.; Denton, C.P.; Hoyles, R.K.; Parfrey, H.; Renzoni, E.A.; Kokosi, M.; et al. Rituximab versus Intravenous Cyclophosphamide in Patients with Connective Tissue Disease-Associated Interstitial Lung Disease in the UK (RECITAL): A Double-Blind, Double-Dummy, Randomised, Controlled, Phase 2b Trial. Lancet Respir. Med. 2023, 11, 45–54.
- Ebata, S.; Yoshizaki, A.; Oba, K.; Kashiwabara, K.; Ueda, K.; Uemura, Y.; Watadani, T.; Fukasawa, T.; Miura, S.; Yoshizaki-Ogawa, A.; et al. Safety and Efficacy of Rituximab in Systemic Sclerosis (DESIRES): A Double-Blind, Investigator-Initiated, Randomised, Placebo-Controlled Trial. Lancet Rheumatol. 2021, 3, e489–e497.
- Morgan, K.; Woollard, C.; Beinart, D.; Host, L.V.; Roddy, J. Rituximab Treatment for Systemic Sclerosis-associated Interstitial Lung Disease: A Case Series of 13 Patients. Intern. Med. J. 2023, 53, 1147–1153.
- Yoshifuji, H.; Yomono, K.; Yamano, Y.; Kondoh, Y.; Yasuoka, H. Role of Rituximab in the Treatment of Systemic Sclerosis: A Literature Review. Mod. Rheumatol. 2023, 33, 1068–1077.
- Shipa, M.; Embleton-Thirsk, A.; Parvaz, M.; Santos, L.R.; Muller, P.; Chowdhury, K.; Isenberg, D.A.; Doré, C.J.; Gordon, C.; Ehrenstein, M.R. Effectiveness of Belimumab After Rituximab in Systemic Lupus Erythematosus. Ann. Intern. Med. 2021, 174, 1647–1657.
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A Moving Target in Immunotherapy. Blood 2018, 131, 58–67.
- Pombo-Suarez, M.; Gomez-Reino, J.J. Abatacept for the Treatment of Rheumatoid Arthritis. Expert Rev. Clin. Immunol. 2019, 15, 319–326.
- Pimentel-Quiroz, V.R.; Ugarte-Gil, M.F.; Alarcón, G.S. Abatacept for the Treatment of Systemic Lupus Erythematosus. Expert Opin. Investig. Drugs 2016, 25, 493–499.
- Chung, L.; Spino, C.; McLain, R.; Johnson, S.R.; Denton, C.P.; Molitor, J.A.; Steen, V.D.; Lafyatis, R.; Simms, R.W.; Kafaja, S.; et al. Safety and Efficacy of Abatacept in Early Diffuse Cutaneous Systemic Sclerosis (ASSET): Open-Label Extension of a Phase 2, Double-Blind Randomised Trial. Lancet Rheumatol. 2020, 2, e743–e753.
- Khanna, D.; Spino, C.; Johnson, S.; Chung, L.; Whitfield, M.L.; Denton, C.P.; Berrocal, V.; Franks, J.; Mehta, B.; Molitor, J.; et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheumatol. 2020, 72, 125–136.
- Bezalel, S.; Guri, K.M.; Elbirt, D.; Asher, I.; Sthoeger, Z.M. Type I Interferon Signature in Systemic Lupus Erythematosus. Isr. Med. Assoc. J. 2014, 16, 246–249.
- Lin, C.M.A.; Isaacs, J.D.; Cooles, F.A.H. Role of IFN-α in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2024, 26, 37–52.
- Casey, K.A.; Guo, X.; Smith, M.A.; Wang, S.; Sinibaldi, D.; Sanjuan, M.A.; Wang, L.; Illei, G.G.; White, W.I. Type I Interferon Receptor Blockade with Anifrolumab Corrects Innate and Adaptive Immune Perturbations of SLE. Lupus Sci. Med. 2018, 5, e000286.
- Liu, Z.; Cheng, R.; Liu, Y. Evaluation of Anifrolumab Safety in Systemic Lupus Erythematosus: A Meta-Analysis and Systematic Review. Front. Immunol. 2022, 13, 996662.
- Kakkar, V.; Assassi, S.; Allanore, Y.; Kuwana, M.; Denton, C.P.; Khanna, D.; Del Galdo, F. Type 1 Interferon Activation in Systemic Sclerosis: A Biomarker, a Target or the Culprit. Curr. Opin. Rheumatol. 2022, 34, 357–364.
- Tovey, M.G.; Lallemand, C. Immunogenicity and Other Problems Associated with the Use of Biopharmaceuticals. Ther. Adv. Drug Saf. 2011, 2, 113–128.
- Maini, R.N.; Taylor, P.C.; Szechinski, J.; Pavelka, K.; Bröll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J.; et al. Double-blind Randomized Controlled Clinical Trial of the Interleukin-6 Receptor Antagonist, Tocilizumab, in European Patients with Rheumatoid Arthritis Who Had an Incomplete Response to Methotrexate. Arthritis Rheum. 2006, 54, 2817–2829.
- Gabay, C.; Emery, P.; van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab Monotherapy versus Adalimumab Monotherapy for Treatment of Rheumatoid Arthritis (ADACTA): A Randomised, Double-Blind, Controlled Phase 4 Trial. Lancet 2013, 381, 1541–1550.
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Wagner, B.; Zucchetto, M.; Raghu, G.; Martinez, F.J.; Goldin, J.; Siegel, J.; Denton, C.P. Long-Term Safety and Efficacy of Tocilizumab in Early Systemic Sclerosis–Interstitial Lung Disease: Open-Label Extension of a Phase 3 Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 674–684.
- Roofeh, D.; Lin, C.J.F.; Goldin, J.; Kim, G.H.; Furst, D.E.; Denton, C.P.; Huang, S.; Khanna, D. Tocilizumab Prevents Progression of Early Systemic Sclerosis–Associated Interstitial Lung Disease. Arthritis Rheumatol. 2021, 73, 1301–1310.
- Chaoyi, M.; Shrestha, B.; Hui, L.; Qiujin, D.; Ping, F. Tocilizumab Therapy for Persistent High-Grade Fever in Systemic Lupus Erythematosus: Two Cases and a Literature Review. J. Int. Med. Res. 2022, 50, 030006052210885.
- Yadav, S.; Sharma, V.; Balakrishnan, C. Tocilizumab Therapy for Treatment-Resistant Systemic Lupus Erythematosus with Elevated IL-6 and CRP Levels: A Case Report. SN Compr. Clin. Med. 2023, 5, 199.
