Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Lara Saftić Martinović.
Addiction is a complex brain disease influenced by genetic, environmental, and neurological factors. Psychostimulants, cocaine, and methamphetamine influence different cell types in different brain regions, with a focus on the neurons responsible for rewarding effects in the nucleus accumbens (NAc) and ventral tegmental area (VTA). Known markers for psychostimulant-induced neuronal plasticity in combination with droplet-based high-throughput single-cell sequencing divided the heterogeneity of cell populations in NAc and VTA into clusters, where all cells of the same type do not respond equally to exposure to psychostimulants.
- addiction
- psychostimulants
- psychostimulant-induced neuronal plasticity
1. Introduction
Behaviorally, addiction is defined as compulsive drug seeking and use despite negative consequences [1]. The underlying molecular mechanisms primarily involve the brain reward system, associated with dopamine release [2,3,4][2][3][4]. Acute drug exposure disrupts neurotransmitter signaling, localization, metabolism, and synthesis, resulting in neuroadaptive changes that contribute to the development of addiction [5,6][5][6]. Prolonged drug exposure induces neuroplastic changes, altering the structure and function of neurons and influencing synaptic connections, thus impacting reward neuronal circuitry and resulting in long-term behavioral changes [7,8][7][8].
Drugs of abuse are a diverse group of compounds classified into various categories based on their pharmacological effects and molecular mechanisms of action. Cocaine (COC) and METH are central nervous system stimulants that increase dopamine release in brain regions of the mesolimbic circuit, influencing reward and cognitive functions [10][9]. Although COC and METH have similar behavioral and physiological effects, there are some major differences. COC is quickly removed and completely metabolized in the body, while METH has a longer duration of action due to slower metabolism and remains unchanged in the brain longer than COC, leading to prolonged stimulant effects [11][10]. Both COC and METH are highly addictive stimulants that are widely recognized as one of the most abused drugs in the world [12,13][11][12]. Key brain regions involved in reward processing, influenced by COC and METH, include the nucleus accumbens (NAc) and ventral tegmental area (VTA). Long-term changes induced by psychostimulant exposure in these regions involve alterations in gene expression and cellular physiology [14,15][13][14]. Psychostimulant-induced synaptic plasticity is linked to the known mechanism of phosphorylation of extracellular signal-regulated kinases (ERK1/2) and cyclic adenosine 3′,5′-monophosphate (cAMP)-response element-binding protein (CREB), with cAMP-dependent protein kinase (PKA) also playing a role in drug-induced memory formation [16,17,18,19][15][16][17][18].
While some people only use drugs for experimentation and never develop an addiction, others develop an addiction after being exposed to psychoactive substances on a regular basis. Understanding the molecular basis for this phenomenon requires a complex interplay of genetic, environmental, and neurobiological factors. Individual variability and the dynamic nature of drug responses both influence the molecular mechanisms involved in the cascade of events that affect neurotransmitter release, receptor activation, and intracellular signaling pathways. Individual vulnerability and susceptibility to addiction were studied in a mouse model, and it was discovered that in wild-type populations, voluntary oral methamphetamine consumption can undergo bidirectional selective breeding, producing two strings with high and low preference for METH [20][19]. Using a selective breeding approach, candidate genes can be identified in the absence of METH exposure. By using RNA-Seq to selectively breed low- and high-METH-preference mice, it was discovered that the trace amine-associated receptor 1 (Taar1) gene plays an important role [21][20].
Previous studies on molecular changes associated with addiction have primarily focused on candidate genes identified by proteomic or genetic techniques using tissue extracts from drug-exposed individuals. With this approach, it is not possible to distinguish in which cells the genetic changes originate, whether they occur at the gene or protein level and whether the observed changes are time-dependent or stable. To explain the complexities of these influences, it is necessary to refine the accepted central dogma, foundational to our understanding of genetic information flow, by applying epigenetics and epitranscriptomics to gene regulation (Figure 1).
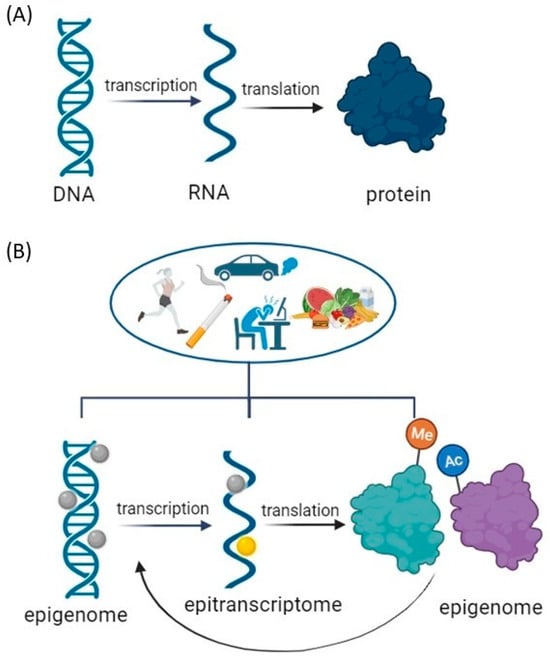
Figure 1. Modifications to the Central Dogma of Molecular Biology: Gene Regulation Modulation by Environmental Factors. (A) Central Dogma: a theory stating that genetic information flows only in one direction, from DNA to RNA to protein, or RNA directly to protein. (B) Environmental Modulation of Central Dogma: Impact on DNA Unfolding, Transcription, and Translation Processes. Epigenome modification = DNA methylation or acetylation and post-translational modifications of histone tails that include phosphorylation, ubiquitination, acetylation, and methylation. Epitranscriptome modifications = RNA modifications primary messenger RNA (mRNA) modifications: N1-methyladenosine (m1A), N6–methyladenosine (m6A), 5–methylcytosine (m5C), pseudouridine (Ψ), and others. Created with BioRender.com (accessed on 11 December 2023).
2. Single-Cell Sequencing Techniques
Traditional classification divides cell types based on morphology rather than molecular features [31,32][21][22]. Despite the fact that cells have almost identical genotypes, traditional RNA sequencing gives an average expression profile from a population of cells, ignoring cell-to-cell variability and transcriptome information from a subset of active genes [33,34,35][23][24][25]. Single-cell RNA sequencing (scRNA-seq) is used for genetic profiling of cells that require prior separation of one cell type from a cell suspension using a fluorescence-activated cell sorting and flow cytometer [36][26]. This approach requires a priori knowledge of a cell-type-specific marker, which is dependent on the availability of appropriate antibodies [37][27]. Great progress in scRNA-seq was made by applying high-throughput droplet-based techniques, which do not require prior separation of the cells from the cell suspension, namely, InDrop [38][28], Drop-seq [39][29], and 10× Chromium [40][30], supporting cost-effective capture and library production for thousands to millions of cells and allowing examination of gene expression heterogeneity among individual cells (Figure 2).
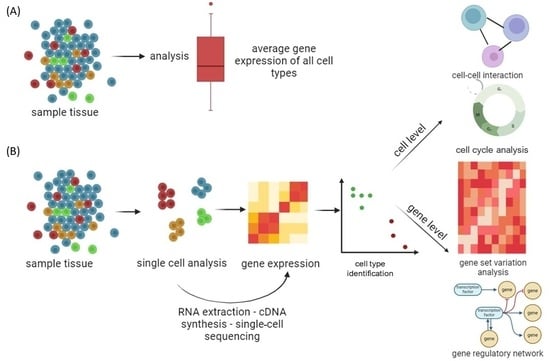

Figure 2. Genomics, transcriptomics, and epigenomics technologies focused on the characterization of individual cells from tissue samples. (A) Conventional massive population sequencing provides average expression signals for different cells, ignoring cell-to-cell variability. (B) By applying high-throughput droplet-based scRNA-seq to complex cell populations, it is possible to uncover different cell types and interactions between cells, follow the development of distinct cell lineages, track changes in gene expression, and identify regulatory relationships between genes. Created with BioRender.com (accessed on 11 December 2023).
2.1. Application of Single-Cell Sequencing in Addiction Research
By defining region-specific molecular signatures and neuronal circuits involved in addictive behaviors [48[31][32][33],49,50], droplet-based high-throughput scRNA-seq enabled researchers to compare gene expression profiles in different brain regions involved in addiction [51][34]. At the molecular level, scRNA-seq of individual neurons identifies specific neuronal subtypes associated with reward, reinforcement, and addiction processes [52][35], whereas transcriptome analysis identifies distinct transcriptional signatures associated with addiction-related behaviors [53][36]. Understanding how individual cells within a population exhibit different sensitivities or adaptations to drug use is crucial, while interactions between the nervous and immune systems must be integrated in this aspect.
2.1.1. Identifying Cell Populations by Molecular Clustering
scRNA-seq has been shown to be an effective tool for characterizing cellular diversity in brain regions associated with the reward system, such as the NAc and VTA [54,55][37][38]. The NAc is a brain region primarily composed of two main types of cells, namely, Medium Spiny Neurons (MSNs) and interneurons. In NAc, clustering analysis revealed nine major cell populations, four neuronal and five non-neuronal [54][37]. Using this approach, in the NAc, novel subpopulations of interneurons and MSNs were identified [52][35]. scATAC-seq has been used to map cell-type-specific differences in chromatin accessibility in the NAc, providing insights into the epigenomic landscape of this brain region [55][38]. Moreover, the characterization of γ-Aminobutyric Acid (GABA) MSNs and the discovery of notable variations in receptor expression patterns and MSN activation within NAc subterritories have highlighted the anatomical and functional heterogeneity of the NAc [56][39]. The ventral tegmental area (VTA) is best known for containing dopaminergic neurons associated with reward and motivation. scRNA-seq identified in VTA selective markers for dopamine and combinatorial neurons revealed expression profiles for drugs of abuse receptors and population-specific enrichment of genes associated with brain disorders [57][40]. This comprehensive molecular characterization highlights the heterogeneity of the NAc and VTA cell population, providing a valuable resource for future research into VTA and NAc gene expression and its implications for reward-related behaviors such as addiction.
By applying scRNAseq in the study of acute COC administration in rodents, distinct neuronal clusters within the NAc were discovered. These clusters exhibited known markers associated with two main types of MSNs: dopamine receptor D1–positive (Drd1-MSNs) and dopamine receptor D2–positive (Drd2-MSNs) [58][41]. The same neuronal substrate that allows drugs of abuse to access Drd1 and Drd2 MSNs in NAc has been confirmed to augment and corrupt a shared pathway that normally serves physiological needs [59][42].
METH is another commonly abused psychostimulant that induces synaptic plasticity and pathological memory enhancement [61][43]. Epigenetics plays an important role in regulating METH addiction [62][44]. METH modulates dopamine (DA), norepinephrine (NE), serotonin, glutamate (Glu), and GABA neurotransmitters in the medial prefrontal cortex (mPFC), the VTA, and the NAc through histone acetylation, methylation, micro RNAs (miRNAs), and ubiquitination. These epigenetic mechanisms do not regulate METH-induced addiction alone but rather collaborate with miRNA regulation of the Ubiquitin proteasome system [62][44]. Cell clustering was performed on bulk RNA data using single-cell RNA data extracted from astrocytes for analysis of differently expressed genes in METH-exposed mice. The NF-κB signaling pathway, inflammation, and neurodegeneration were among the considerably enriched pathways found in the analysis. Immune infiltration analysis showed that the METH group had significantly lower neutrophil infiltration and significantly higher monocyte, T, and NK cell infiltration [63][45]. Strong inflammatory responses occur early in METH withdrawal, and they decrease as withdrawal time increases.
2.1.2. Identifying Transcriptome Mechanism
By analyzing single-cell transcriptional responses in the prefrontal cortex (PFC) cells of mice undergoing COC self-administration, specific cell types that express genes associated with COC addiction were identified, namely, ∆FosB, Methyl CpG binding protein 2 (MeCP2) and brain-derived neurotrophic factor (BDNF) [68][46]. Following COC administration, ∆FosB was reported to also be expressed in the VTA with dopamine neurons projecting to the NAc [69][47], MeCP2 was reported to be altered in the NAc [70][48], and BDNF expression played a role in the VTA-NAc pathway [71][49]. These genes were selectively expressed in excitatory neurons, inhibitory neurons, and non-neuronal glial cells while showing varied expression levels over the stages of COC addiction [68][46]. Reactive oxygen species and oxidative stress in striatal regions are known to be elevated by COC, and this effect is further compounded by elevated glutamate release and excessive dopamine levels. The potential involvement of both cell types in the regulation of conserved gene networks was revealed by integrating human transcriptomes with the Drd1 and Drd2 MSN transcriptome data from mice. Numerous transcription factors that are predicted to be upstream of the abnormalities in gene expression linked to addiction were identified through this analysis. The majority are shared between the NAc and dorsum striatum. Notably, several of these predicted upstream transcription factors have been implicated previously in COC addiction in rodent models; in particular, Activator protein 1 (AP-1) (FOS/JUN) family, EGR family, NFκB, E2F, and several nuclear hormone receptors cells [72][50]. In response to acute and chronic METH, mice hippocampi exhibit significant volumetric atrophy compared to controls. The genes involved in cytoskeleton organization and phagocytosis were downregulated in the acute METH-treated group compared to the control group. In the group receiving long-term METH treatment, genes linked to synaptic transmission, neuron differentiation regulation, and neurogenesis regulation were downregulated [73][51]. Drd1 overstimulation after METH exposure induces metabolic changes and transcriptional pathways, switching gene expression and neuronal phenotype underlying addictive behavior [74][52]. PKA phosphorylates voltage-dependent ion channels, GLUT receptors, transcription factors, and epigenetic enzymes involved in synaptic plasticity as naturally occurring in normal striatum. When the Drd1 is activated, PKA activates mitogen-activated protein kinases (MAPKs) and extracellular signal-regulated kinases 1/2 (ERK1/2) [75][53]. Nuclear receptors, CREB, Elk-1, and H3 histones are all phosphorylated by ERK1/2 upon translocation to the nucleus, where they control the expression of certain genes [76][54]. The DA- and cAMP-regulated phosphoprotein (DARPP-32) is a key substrate of DRD1/PKA signaling in the striatum [77][55]. METH consumption results in oxidative stress in the DA terminals due to excess of free DA undergoing oxidative metabolism and autoxidation, intracellularly and extracellularly. Along with hydrogen peroxide and reactive oxygen species (ROS) production, toxic DA metabolites like quinones and 3,4-Dihydroxyphenylacetaldehyde promote structural modifications of proteins. METH influences other cell organelles, endoplasmic reticulum, and mitochondria, leading to neurotoxic effects, while ROS accumulation leads to misfolded and insoluble proteins and severed organelles, decomposed by cell-clearing mechanisms of autophagy and ubiquitin proteasome [78][56].3. Epigenetic Mechanisms and Psychostimulant Addiction
3.1. Cocaine-induced Epigenetic Modifications
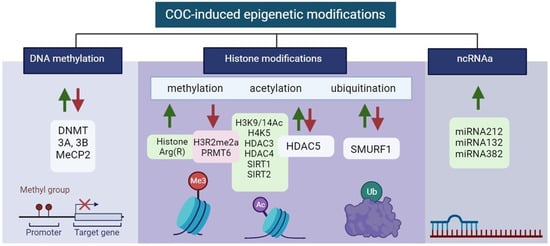
Epigenetic modifications in the control of gene expression of specific brain regions in COC addiction are summarized in Figure 3.
Figure 3. Illustration of aberrant alterations in COC-induced epigenetic modifications. Green and red arrows indicate upregulation and downregulation, respectively. Created with BioRender.com (accessed on 13 December 2023).
3.1.1. DNA Methylation
Environmental stimuli in COC exposure can be translated to changes in gene expression and phenotypes that, through enzymatic modifications to DNA sequences, create long-lasting behavioral phenotypes. These methylated DNA regions are bound by methyl-binding domain-containing proteins such as methyl CpG-binding protein 2 (MeCP2), which is important for recruiting co-repressors such as methyltransferases to the gene promoter [100][57]. DNA methylation is critical for imprinting, X chromosome inactivation, and cell differentiation. Therefore, it is important that it can be altered at specific loci in germ cells by exposure to environmental factors such as toxins [101][58] and stress [102][59], and can therefore be inherited by offspring over multiple generations. Acute cocaine administration increases DNA methylation and DNMT3A and DNMT3B levels, while the binding of MeCP2 to specific gene promoters can decrease gene expression in the NAc [103][60].3.1.2. Histone Modifications
Histones Methylation
Histones H3 and H4 can have their lysine or arginine residues methylated, with varying effects on transcription. While lysine methylation is connected to both transcriptional activation and repression depending on the methylation site, arginine methylation encourages transcriptional activation [106][61]. This adaptability could be explained by the fact that, in contrast to acetylation, methylation has no effect on histone charge or histone–DNA interactions directly. H3 methylation increases the gene transcription, while lysine (K) methylation on H3K9Me3 and H3K27Me3 at specific gene regions represses transcription [107][62].Histones Acetylation
COC administration leads to significant acetylation of histones H3 and H4 [109][63]. H3K9, H3K14, H4K5, H4K8, H4K12, and H4K16 have emerged as the most extensively researched acetylation sites in the context of COC exposure [90][64]. Histone acetylation in the NAc is associated with CREB-binding protein (CBP), a histone acetyltransferase [110][65]. Repeated exposure to COC leads to a dual effect on histone acetylation, resulting in H3Ac/H4Ac-increased and -decreased acetylation of gene promoters, attributed to the hypoacetylation of histones H3 and H4 [109][63]. In the VTA during COC withdrawal, there is an observed enrichment of CBP in the promoter region of BDNF leading to an elevated level of histone acetylation, particularly H3K9/14Ac, at the Bdnf promoter [111][66].Histones Ubiquitination
The ubiquitin-proteasome system (UPS), one of the epigenetic hallmarks, is a multifaceted network of ubiquitin ligases and proteasome structures that controls synaptic and epigenetic plasticity [121][67] and is also involved in memory processes and substance use disorders [122][68]. E3 ubiquitin-protein ligase SMURF1 is a key mediator of neuroadaptations in the nucleus accumbens that follow COC exposure and mediates cue-induced COC seeking during withdrawal [123][69]. All of these results point to the possibility that the SMURF1–SMAD1/5–RUNX2 pathway functions as a crucial transcriptional regulator that modifies plasticity after COC self-administration. The finding that RUNX2 and SMAD1/5 are upregulated in the NAc following COC self-administration allowed researchers to investigate RUNX2 binding to target genes that had previously been connected to COC plasticity. RUNX2 is found to bind at AP-1 sites and to the promoters of these genes following COC self-administration, indicating that it may be a master regulator of several pathways regulating COC-induced plasticity [124][70]. There is a critical need for new research to identify novel potential therapeutic targets in order to develop effective treatments for COC use disorder.3.1.3. ncRNAs
The most commonly investigated ncRNAs involved in epigenome modifications in COC addiction are miRNAs. They can regulate COC intake and potentially have an impact on developing compulsive consumption of the drug. The mechanisms that stand by miR-212 influences COC intake were also a point of exploration. The cAMP signaling cascade strongly activates the miR-212/132 gene cluster, with CREB upregulating both miRNAs. It was found that the pathway of miR-212 regulation dramatically boosts CREB signaling in cultured cells and also in the striatum of rats [104][71]. miR-206 has been shown to negatively regulate BDNF expression, which is known to be important for the motivational effects of COC and other addictive substances [126][72].3.2. Methamphetamine-Induced Epigenetic Modifications
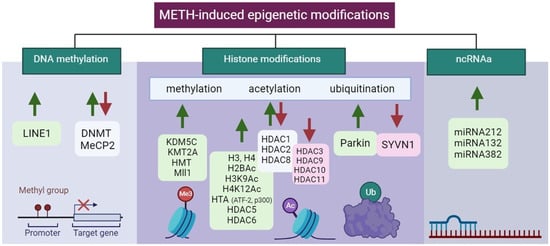
Epigenetic modifications in the control of gene expression of specific brain regions in METH addiction are summarized in Table 2 and Figure 4.
Figure 4. Illustration of aberrant alterations in METH-induced epigenetic modifications. Green and red arrows indicate upregulation and downregulation, respectively. Created with BioRender.com (accessed on 13 December 2023).
3.2.1. DNA Methylation
DNA methylation levels have been found to be changed in METH addicts [129][73], and moreover, this also occurs in their offspring [130][74]. BDNF methylation was increased in the PFC of METH-addicted rats and patients but was decreased in the hippocampus of rats [131][75]. The neurotoxic effects of METH exposure were partly caused by a decrease in BDNF expression [131,132][75][76].
3.2.2. Histone Modifications
Histones Methylation
According to recent research, epigenetic mechanisms that mediate drug-induced transcriptional and behavioral changes induced by METH consumption are mostly caused by histone modification [139][77]. Indeed, it was shown that METH induced H3 methylation through increased tri-methylation of histone H3 at lysine 4 (H3K4me3) at the promoter site of chemokine receptor 2 associated with behavioral sensitization in mice [140][78].Histones Acetylation
Similar to COC, METH induces alterations in the acetylation levels of histones H3 and H4, influencing the expression of various enzymes in different brain regions [141][79]. In NAc, METH exposure leads to differential acetylation changes on various histone lysine residues by regulating the protein levels of histone deacetylases [142][80]. Acute METH exposure results in decreased H3 acetylation (H3K9Ac and H3K18Ac) and increased H4 acetylation (H4K5Ac, H4K8Ac, and H4K16Ac) [143][81]. Chronic METH causes a reduction in histone H4 acetylation (H4K5Ac, H4K12Ac, and H4K16Ac) at glutamate (GLUT) receptor promoters, impacting the expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors, leading to oxidation, excitotoxicity, and neuroinflammation [144][82].Histones Ubiquitination
Neurotransmitter excitation and synaptic plasticity in brain disorders associated with drug addiction have been connected to the UPS, an enzymatic complex that controls proteolysis and turnover. Both pre- and post-synaptic neurons in the DA circuitry are impacted by UPS inhibition. Through endocytic internalization and degradation, the UPS reduces D1/D2-like DRs and Alphaamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [150][83]. The UPS regulates the presynaptic release of Glu via both D1-like and D2-like receptors, which controls DA transmission [151][84], also influencing postsynaptic plasticity [152,153][85][86]. As a result, the DA and Glu signaling pathways interact with UPS substrates [121][67]. METH, despite being an extremely powerful DA releaser, impairs UPS activity, which is largely due to dopamine. As a result, pre- and post-synaptic neurons in the dopamine circuitry are extremely vulnerable to UPS inhibition [150][83]. Parkin promotes the ubiquitination of substrate proteins, which aids in their degradation.3.3. ncRNAs
Similarly, as in COC addiction, epigenetic modifications in METH addiction involving ncRNA predominantly include miRNAs. Using the KEGG pathway analysis, miRNA-regulated genes were found to be involved in vesicular transport, METH addiction, the cyclic guanosine monophosphate cGMP-protein kinases G (PKG) signaling pathway, the dopaminergic synapse, and the GABAergic synapse [157][87]. In genome-wide transcriptional profiling, the expression of multiple miRNAs is increased in the central amygdala alongside molecules related to METH addiction [155][88]. METH increased the levels of miRNAs 237, 296, and 501 in the NAc. MiRNAs in the NAc regulate Wnt signaling and axon guidance genes [158][89]. MiR-128 influenced METH-induced behavioral sensitization by altering synaptic plasticity-related molecules in the NAc [159][90]. METH-induced locomotor sensitization is disrupted by Ago2-dependent miRNAs in the NAc. These Ago2/miR-3068-5p effects occur in conjunction with the glutamate receptor, GluN1/Grin1 [138][91]. METH can increase the expression of miRNAs in the striatum, which harmed motor coordination and reduced striatal volume and dendritic length [160][92].4. Epitranscriptomics and Psychostimulant Addiction
Epigenetic modifications to DNA and histones regulate gene transcription, whereas epitranscriptomic post-transcriptional RNA modifications influence gene expression [165][93]. The most common mRNA modifications are N1-methyladenosine (m1A), N6-methyladenosine (m6A), 5-methylcytosine (m5C), pseudouridine (Ψ), and others [166][94]. m6A modification is one of the most abundant and reversible epitranscriptomic modifications, mediated by a set of proteins, which include methyltransferases (‘writers’), demethylases (‘erasers’), and m6A-binding proteins (‘readers’) [167][95]. m6A methylation is associated with the control of mRNA metabolism, splicing, export, stability, translation, and degradation [168,169][96][97]. Out of all tissues in the body, the brain has the highest abundance of m6A methylation, which is developmentally decreasing [168][96]. In the brain, m6A methylation regulates neuronal transcripts and neuronal activity. Aside from its role in neuronal development [30][98], m6A modification is essential for the process of axon regeneration [170][99]. Methyltransferases like-3 (METTL3) and like-14 (METTL14) [171[100][101][102],172,173], along with other proteins needed for m6A deposition, such as Wilms’ tumor 1-associating protein (WTAP) [174][103] and RNA binding protein 15 (Rbm15) [175][104], form a stable protein complex that catalyzes m6A modification. Because they deposit RNA methylation modifications, these methyltransferases are collectively known as “writers”. Fat mass obesity-associated protein (FTO) and alkB homologue 5 (ALKBH5) are two demethylases from the family of α-ketoglutarate dependent dioxygenases that can reverse m6A modification because it is dynamically regulated [176,177][105][106]. Demethylases are known as “erasers” because they remove RNA methylation modifications. Posttranscriptional, site-specific adenosine-to-inosine base conversions, known as RNA editing, contribute to gene expression diversity and are catalyzed by Adenosine deaminases acting on RNA (ADARs) [178][107]. Pseudouridine Synthase 7 (PUS7) is one of the major mRNA-modifying enzymes leading to pseudouridine (Ψ), a ubiquitous RNA modification [179][108]. Both ADAR and PUS7 can lead to further mRNA modifications. m6A modifications are in direct connection to the so-called m6A “reader” proteins, which recognize the modified site. The proteins with YTH domains, which can specifically bind m6A through their YTH domain, are the most well-studied m6A readers. Fragile X mental retardation protein (FMRP) was also reported to be m6A reader and plays critical roles in synaptic plasticity and neuronal development. The identification of methylated nucleosides (m6A, m5C, m1A) is performed using immunoprecipitation and their variants using antibodies against methylated nucleosides or associated proteins methyltransferases, demethylases, and binding proteins. After fragmenting the RNA, fragments containing modified nucleosides are enriched before sequencing. The immunoprecipitate is analyzed using next-generation sequencing (NGS) to identify and map the modification [180][109]. The connection between epigenetic regulation and m6A RNA modification was associated with histone H3 trimethylation at Lys36 (H3K36me3), a marker for transcription elongation, which guides m6A deposition globally connected through METTL14 (DOI: 10.1038/s41586-019-1016-7). m6A modifications are mainly associated with neuronal plasticity in the brain, which is a consequence of learning and memory, and most of the literature is based on this line of research together with neurodegenerative disorders [176,181,182][105][110][111]. Indeed, deficiency in m6A-dependent pathways significantly impairs neuronal function including dopamine signaling and dopamine-dependent learning. Lowering neuronal m6A by overexpressing FTO or by adding m6A inhibitor led to the induction of N-methyl-d-aspartate (NMDA) receptor 1 expression, elevated oxidative stress, and Ca2+ influx, resulting in dopaminergic neuron apoptosis [183][112]. In addition, it was shown that the overexpression of FTO delays the dephosphorylation of CREB, increases the expression of the CREB, and targets neuropeptide receptor 1 (NPY1R) and BDNF known to regulate food intake and energy homeostasis [184][113]. FTO affects dopamine (D2)-dependent responses to reward learning in meso-striato-prefrontal regions, suggesting a mechanism by which genetic predisposition alters reward processing not only in obesity but also in other disorders with altered D2R-dependent impulse control, such as addiction [185][114].References
- Steketee, J.D.; Kalivas, P.W. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacol. Rev. 2011, 63, 348–365.
- Robbins, T.W.; Everitt, B.J. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996, 6, 228–236.
- Robinson, T.E.; Berridge, K.C. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3137.
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. Adv. Exp. Med. Biol. 2021, 1344, 57–69.
- Rezayof, A.; Ghasemzadeh, Z.; Sahafi, O.H. Addictive drugs modify neurogenesis, synaptogenesis and synaptic plasticity to impair memory formation through neurotransmitter imbalances and signaling dysfunction. Neurochem. Int. 2023, 169, 105572.
- Peters, K.Z.; Cheer, J.F.; Tonini, R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci. 2021, 44, 464–477.
- Lüscher, C.; Malenka, R.C. Drug-evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron 2011, 69, 650–663.
- Kutlu, M.G.; Gould, T.J. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn. Mem. 2016, 23, 515–533.
- Perry, A.N.; Westenbroek, C.; Becker, J.B. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE 2013, 8, e79465.
- Chiu, V.M.; Schenk, J.O. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr. Drug Abus. Rev. 2012, 5, 227–242.
- Chomchai, C.; Chomchai, S. Global patterns of methamphetamine use. Curr. Opin. Psychiatry 2015, 28, 269–274.
- Gangu, K.; Bobba, A.; Basida, S.D.; Avula, S.; Chela, H.; Singh, S. Trends of Cocaine Use and Manifestations in Hospitalized Patients: A Cross-Sectional Study. Cureus 2022, 14, e22090.
- Chen, G.; Lai, S.; Bao, G.; Ke, J.; Meng, X.; Lu, S.; Wu, X.; Xu, H.; Wu, F.; Xu, Y.; et al. Distinct reward processing by subregions of the nucleus accumbens. Cell Rep. 2023, 42, 112069.
- Becker-Krail, D.D.; Walker, W.H.; Nelson, R.J. The Ventral Tegmental Area and Nucleus Accumbens as Circadian Oscillators: Implications for Drug Abuse and Substance Use Disorders. Front. Physiol. 2022, 13, 886704. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2022.886704 (accessed on 11 December 2023).
- Lee, A.M.; Messing, R.O. Protein Kinases and Addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 22–57.
- Amaral, I.M.; Scheffauer, L.; Hofer, A.; El Rawas, R. Protein kinases in natural versus drug reward. Pharmacol. Biochem. Behav. 2022, 221, 173472.
- Shi, X.; McGinty, J.F. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. J. Neurochem. 2007, 103, 706–713.
- Jia, W.; Kawahata, I.; Cheng, A.; Fukunaga, K. The Role of CaMKII and ERK Signaling in Addiction. Int. J. Mol. Sci. 2021, 22, 3189.
- Wheeler, J.M.; Reed, C.; Burkhart-Kasch, S.; Li, N.; Cunningham, C.L.; Janowsky, A.; Franken, F.H.; Wiren, K.M.; Hashimoto, J.G.; Scibelli, A.C.; et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009, 8, 758–771.
- Hitzemann, R.; Iancu, O.D.; Reed, C.; Baba, H.; Lockwood, D.R.; Phillips, T.J. Regional Analysis of the Brain Transcriptome in Mice Bred for High and Low Methamphetamine Consumption. Brain Sci. 2019, 9, 155.
- Zeng, H.; Sanes, J.R. Neuronal cell-type classification: Challenges, opportunities and the path forward. Nat. Rev. Neurosci. 2017, 18, 530–546.
- Arendt, D. The evolution of cell types in animals: Emerging principles from molecular studies. Nat. Rev. Genet. 2008, 9, 868–882.
- Zeng, H. What is a cell type and how to define it? Cell 2022, 185, 2739–2755.
- Miller, J.A.; Gouwens, N.W.; Tasic, B.; Collman, F.; van Velthoven, C.T.; Bakken, T.E.; Hawrylycz, M.J.; Zeng, H.; Lein, E.S.; Bernard, A. Common cell type nomenclature for the mammalian brain. eLife 2020, 9, e59928.
- Gupta, A.D.; Asan, L.; John, J.; Beretta, C.; Kuner, T.; Knabbe, J. Accurate classification of major brain cell types using in vivo imaging and neural network processing. PLoS Biol. 2023, 21, e3002357.
- O’Donnell, E.A.; Ernst, D.N.; Hingorani, R. Multiparameter flow cytometry: Advances in high resolution analysis. Immune Netw. 2013, 13, 43–54.
- Baron, C.S.; Barve, A.; Muraro, M.J.; van der Linden, R.; Dharmadhikari, G.; Lyubimova, A.; de Koning, E.J.; van Oudenaarden, A. Cell Type Purification by Single-Cell Transcriptome-Trained Sorting. Cell 2019, 179, 527–542.e19.
- Klein, A.M.; Mazutis, L.; Akartuna, I.; Tallapragada, N.; Veres, A.; Li, V.; Peshkin, L.; Weitz, D.A.; Kirschner, M.W. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 2015, 161, 1187–1201.
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214.
- Zheng, G.X.Y.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049.
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773.
- Uhl, G.R.; Koob, G.F.; Cable, J. The neurobiology of addiction. Ann. N. Y. Acad. Sci. 2019, 1451, 5–28.
- Poisson, C.L.; Engel, L.; Saunders, B.T. Dopamine Circuit Mechanisms of Addiction-Like Behaviors. Front. Neural Circuits 2021, 15, 125. Available online: https://www.frontiersin.org/articles/10.3389/fncir.2021.752420 (accessed on 11 December 2023).
- Srinivasan, C.; Phan, B.N.; Lawler, A.J.; Ramamurthy, E.; Kleyman, M.; Brown, A.R.; Kaplow, I.M.; Wirthlin, M.E.; Pfenning, A.R. Addiction-Associated Genetic Variants Implicate Brain Cell Type- and Region-Specific Cis-Regulatory Elements in Addiction Neurobiology. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 9008–9030.
- Tran, M.N.; Maynard, K.R.; Spangler, A.; Huuki, L.A.; Montgomery, K.D.; Sadashivaiah, V.; Tippani, M.; Barry, B.K.; Hancock, D.B.; Hicks, S.C.; et al. Single-nucleus transcriptome analysis reveals cell-type-specific molecular signatures across reward circuitry in the human brain. Neuron 2021, 109, 3088–3103.e5.
- Navandar, M.; Martín-García, E.; Maldonado, R.; Lutz, B.; Gerber, S.; de Azua, I.R. Transcriptional signatures in prefrontal cortex confer vulnerability versus resilience to food and cocaine addiction-like behavior. Sci. Rep. 2021, 11, 9076.
- Chen, R.; Blosser, T.R.; Djekidel, M.N.; Hao, J.; Bhattacherjee, A.; Chen, W.; Tuesta, L.M.; Zhuang, X.; Zhang, Y. Decoding molecular and cellular heterogeneity of mouse nucleus accumbens. Nat. Neurosci. 2021, 24, 1757–1771.
- Bhatia, P.; Yang, L.; Luo, J.X.J.; Xu, M.; Renthal, W. Epigenomic profiling of mouse nucleus accumbens at single-cell resolution. Mol. Cell. Neurosci. 2023, 126, 103857.
- Gangarossa, G.; Espallergues, J.; D’Exaerde, A.d.K.; El Mestikawy, S.; Gerfen, C.R.; Hervé, D.; Girault, J.-A.; Valjent, E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front. Neural Circuits 2013, 7, 22.
- Phillips, R.A.; Tuscher, J.J.; Black, S.L.; Andraka, E.; Fitzgerald, N.D.; Ianov, L.; Day, J.J. An atlas of transcriptionally defined cell populations in the rat ventral tegmental area. Cell Rep. 2022, 39, 110616.
- Savell, K.E.; Tuscher, J.J.; Zipperly, M.E.; Duke, C.G.; Phillips, R.A.; Bauman, A.J.; Thukral, S.; Sultan, F.A.; Goska, N.A.; Ianov, L.; et al. A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci. Adv. 2020, 6, eaba4221.
- Tan, B.; Browne, C.J.; Nöbauer, T.; Vaziri, A.; Friedman, J.M.; Nestler, E.J. Drugs of abuse hijack a mesolimbic pathway that processes homeostatic need. bioRxiv 2023, 2023.09.03.556059.
- Čechová, B.; Šlamberová, R. Methamphetamine, neurotransmitters and neurodevelopment. Physiol. Res. 2021, 70, S301–S315.
- Wang, H.; Dong, X.; Awan, M.U.N.; Bai, J. Epigenetic mechanisms involved in methamphetamine addiction. Front. Pharmacol. 2022, 13, 984997. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2022.984997 (accessed on 11 December 2023).
- Li, K.; Ling, H.; Wang, X.; Xie, Q.; Gu, C.; Luo, W.; Qiu, P. The role of NF-κB signaling pathway in reactive astrocytes among neurodegeneration after methamphetamine exposure by integrated bioinformatics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 129, 110909.
- Bhattacherjee, A.; Djekidel, M.N.; Chen, R.; Chen, W.; Tuesta, L.M.; Zhang, Y. Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat. Commun. 2019, 10, 4169.
- Perrotti, L.I.; Bolaños, C.A.; Choi, K.; Russo, S.J.; Edwards, S.; Ulery, P.G.; Wallace, D.L.; Self, D.W.; Nestler, E.J.; Barrot, M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005, 21, 2817–2824.
- Vaillancourt, K.; Ernst, C.; Mash, D.; Turecki, G. DNA Methylation Dynamics and Cocaine in the Brain: Progress and Prospects. Genes 2017, 8, 138.
- Eisch, A.J.; Bolaños, C.A.; de Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry 2003, 54, 994–1005.
- Mews, P.; Cunningham, A.M.; Scarpa, J.; Ramakrishnan, A.; Hicks, E.M.; Bolnick, S.; Garamszegi, S.; Shen, L.; Mash, D.C.; Nestler, E.J. Convergent abnormalities in striatal gene networks in human cocaine use disorder and mouse cocaine administration models. Sci. Adv. 2023, 9, eadd8946.
- Choi, M.R.; Chun, J.-W.; Kwak, S.M.; Bang, S.H.; Jin, Y.-B.; Lee, Y.; Kim, H.-N.; Chang, K.-T.; Chai, Y.G.; Lee, S.-R.; et al. Effects of acute and chronic methamphetamine administration on cynomolgus monkey hippocampus structure and cellular transcriptome. Toxicol. Appl. Pharmacol. 2018, 355, 68–79.
- Ujike, H.; Onoue, T.; Akiyama, K.; Hamamura, T.; Otsuki, S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology 1989, 98, 89–92.
- Gerfen, C.R.; Miyachi, S.; Paletzki, R.; Brown, P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 5042–5054.
- Mizoguchi, H.; Yamada, K.; Mizuno, M.; Mizuno, T.; Nitta, A.; Noda, Y.; Nabeshima, T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004, 65, 1293–1301.
- Valjent, E.; Pascoli, V.; Svenningsson, P.; Paul, S.; Enslen, H.; Corvol, J.-C.; Stipanovich, A.; Caboche, J.; Lombroso, P.J.; Nairn, A.C.; et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. USA 2005, 102, 491–496.
- Limanaqi, F.; Gambardella, S.; Biagioni, F.; Busceti, C.L.; Fornai, F. Epigenetic Effects Induced by Methamphetamine and Methamphetamine-Dependent Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, e4982453.
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476.
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 2010, 5, e13100.
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415.
- Anier, K.; Malinovskaja, K.; Aonurm-Helm, A.; Zharkovsky, A.; Kalda, A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 2450–2461.
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357.
- Feng, J.; Wilkinson, M.; Liu, X.; Purushothaman, I.; Ferguson, D.; Vialou, V.; Maze, I.; Shao, N.; Kennedy, P.; Koo, J.; et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 2014, 15, R65.
- Renthal, W.; Kumar, A.; Xiao, G.; Wilkinson, M.; Covington, H.E.; Maze, I.; Sikder, D.; Robison, A.J.; LaPlant, Q.; Dietz, D.M.; et al. Genome Wide Analysis of Chromatin Regulation by Cocaine Reveals a Novel Role for Sirtuins. Neuron 2009, 62, 335–348.
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705.
- Chrivia, J.C.; Kwok, R.P.S.; Lamb, N.; Hagiwara, M.; Montminy, M.R.; Goodman, R.H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 1993, 365, 855–859.
- Schmidt, H.D.; Sangrey, G.R.; Darnell, S.B.; Schassburger, R.L.; Cha, J.J.; Pierce, R.C.; Sadri-Vakili, G. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J. Neurochem. 2012, 120, 202–209.
- Bach, S.V.; Hegde, A.N. The proteasome and epigenetics: Zooming in on histone modifications. Biomol. Concepts 2016, 7, 215–227.
- Jarome, T.J.; Helmstetter, F.J. The ubiquitin–proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol. Learn. Mem. 2013, 105, 107–116.
- Werner, C.T.; Viswanathan, R.; Martin, J.A.; Gobira, P.H.; Mitra, S.; Thomas, S.A.; Wang, Z.-J.; Liu, J.-F.; Stewart, A.F.; Neve, R.L.; et al. E3 Ubiquitin-Protein Ligase SMURF1 in the Nucleus Accumbens Mediates Cocaine Seeking. Biol. Psychiatry 2018, 84, 881–892.
- Zhang, Y. Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev. 2003, 17, 2733–2740.
- Im, H.-I.; Hollander, J.A.; Bali, P.; Kenny, P.J. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 2010, 13, 1120–1127.
- Tapocik, J.D.; Barbier, E.; Flanigan, M.; Solomon, M.; Pincus, A.; Pilling, A.; Sun, H.; Schank, J.R.; King, C.; Heilig, M. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4581–4588.
- Liu, L.; Luo, T.; Dong, H.; Zhang, C.; Liu, T.; Zhang, X.; Hao, W. Genome-Wide DNA Methylation Analysis in Male Methamphetamine Users With Different Addiction Qualities. Front. Psychiatry 2020, 11, 588229.
- Asaoka, Y.; Won, M.; Morita, T.; Ishikawa, E.; Lee, Y.-A.; Goto, Y. Monoamine and genome-wide DNA methylation investigation in behavioral addiction. Sci. Rep. 2020, 10, 11760.
- Iamjan, S.-A.; Thanoi, S.; Watiktinkorn, P.; Fachim, H.; Dalton, C.F.; Nudmamud-Thanoi, S.; Reynolds, G.P. Changes of BDNF exon IV DNA methylation are associated with methamphetamine dependence. Epigenomics 2021, 13, 953–965.
- Salehzadeh, S.A.; Mohammadian, A.; Salimi, F. Effect of chronic methamphetamine injection on levels of BDNF mRNA and its CpG island methylation in prefrontal cortex of rats. Asian J. Psychiatry 2020, 48, 101884.
- Renthal, W.; Nestler, E.J. Epigenetic mechanisms in drug addiction. Trends Mol. Med. 2008, 14, 341–350.
- Ikegami, D.; Narita, M.; Imai, S.; Miyashita, K.; Tamura, R.; Narita, M.; Takagi, S.; Yokomizo, A.; Takeshima, H.; Ando, T.; et al. Epigenetic modulation at the CCR2 gene correlates with the maintenance of behavioral sensitization to methamphetamine. Addict. Biol. 2010, 15, 358–361.
- Cadet, J.L.; Jayanthi, S. Epigenetic Landscape of Methamphetamine Use Disorder. Curr. Neuropharmacol. 2021, 19, 2060–2066.
- Jayanthi, S.; McCoy, M.T.; Cadet, J.L. Epigenetic Regulatory Dynamics in Models of Methamphetamine-Use Disorder. Genes 2021, 12, 1614.
- Martin, T.A.; Jayanthi, S.; McCoy, M.T.; Brannock, C.; Ladenheim, B.; Garrett, T.; Lehrmann, E.; Becker, K.G.; Cadet, J.L. Methamphetamine Causes Differential Alterations in Gene Expression and Patterns of Histone Acetylation/Hypoacetylation in the Rat Nucleus Accumbens. PLoS ONE 2012, 7, e34236.
- Limanaqi, F.; Busceti, C.L.; Celli, R.; Biagioni, F.; Fornai, F. Autophagy as a gateway for the effects of methamphetamine: From neurotransmitter release and synaptic plasticity to psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2021, 204, 102112.
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Ryskalin, L.; Fornai, F. The effects of proteasome on baseline and methamphetamine-dependent dopamine transmission. Neurosci. Biobehav. Rev. 2019, 102, 308–317.
- Briones-Lizardi, L.J.; Cortés, H.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.J.; Aceves, J.; Erlij, D.; Florán, B. Presynaptic control of -glutamate release by dopamine receptor subtypes in the rat substantia nigra. Central role of D1 and D3 receptors. Neuroscience 2019, 406, 563–579.
- Dong, C.; Bach, S.V.; Haynes, K.A.; Hegde, A.N. Proteasome modulates positive and negative translational regulators in long-term synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 3171–3182.
- Hegde, A.N. Proteolysis, synaptic plasticity and memory. Neurobiol. Learn. Mem. 2017, 138, 98–110.
- Li, H.; Li, C.; Zhou, Y.; Luo, C.; Ou, J.; Li, J.; Mo, Z. Expression of microRNAs in the serum exosomes of methamphetamine-dependent rats vs. ketamine-dependent rats. Exp. Ther. Med. 2018, 15, 3369.
- Cates, H.M.; Li, X.; Purushothaman, I.; Kennedy, P.J.; Shen, L.; Shaham, Y.; Nestler, E.J. Genome-wide transcriptional profiling of central amygdala and orbitofrontal cortex during incubation of methamphetamine craving. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 2426–2434.
- Yang, J.; Li, L.; Hong, S.; Zhang, D.; Zhou, Y. Methamphetamine leads to the alterations of microRNA profiles in the nucleus accumbens of rats. Pharm. Biol. 2020, 58, 797–805.
- Li, J.; Zhu, L.; Su, H.; Liu, D.; Yan, Z.; Ni, T.; Wei, H.; Goh, E.L.; Chen, T. Regulation of miR-128 in the nucleus accumbens affects methamphetamine-induced behavioral sensitization by modulating proteins involved in neuroplasticity. Addict. Biol. 2021, 26, e12881.
- Liu, D.; Liang, M.; Zhu, L.; Zhou, T.-T.; Wang, Y.; Wang, R.; Wu, F.-F.; Goh, E.L.K.; Chen, T. Potential Ago2/miR-3068-5p Cascades in the Nucleus Accumbens Contribute to Methamphetamine-Induced Locomotor Sensitization of Mice. Front. Pharmacol. 2021, 12, 708034. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2021.708034 (accessed on 12 December 2023).
- Chavoshi, H.; Boroujeni, M.E.; Abdollahifar, M.-A.; Amini, A.; Tehrani, A.M.; Moghaddam, M.H.; Norozian, M.; Farahani, R.M.; Aliaghaei, A. From dysregulated microRNAs to structural alterations in the striatal region of METH-injected rats. J. Chem. Neuroanat. 2020, 109, 101854.
- Kan, R.L.; Chen, J.; Sallam, T. Crosstalk between epitranscriptomic and epigenetic mechanisms in gene regulation. Trends Genet. TIG 2022, 38, 182–193.
- Flamand, M.N.; Meyer, K.D. The epitranscriptome and synaptic plasticity. Curr. Opin. Neurobiol. 2019, 59, 41–48.
- Angelova, M.T.; Dimitrova, D.G.; Dinges, N.; Lence, T.; Worpenberg, L.; Carré, C.; Roignant, J.-Y. The Emerging Field of Epitranscriptomics in Neurodevelopmental and Neuronal Disorders. Front. Bioeng. Biotechnol. 2018, 6, 46.
- Chokkalla, A.K.; Mehta, S.L.; Vemuganti, R. Epitranscriptomic regulation by m6A RNA methylation in brain development and diseases. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020, 40, 2331–2349.
- Engel, M.; Chen, A. The emerging role of mRNA methylation in normal and pathological behavior. Genes Brain Behav. 2018, 17, e12428.
- Lence, T.; Akhtar, J.; Bayer, M.; Schmid, K.; Spindler, L.; Ho, C.H.; Kreim, N.; Andrade-Navarro, M.A.; Poeck, B.; Helm, M.; et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 2016, 540, 242–247.
- Weng, Y.-L.; Wang, X.; An, R.; Cassin, J.; Vissers, C.; Liu, Y.; Liu, Y.; Xu, T.; Wang, X.; Wong, S.Z.H.; et al. Epitranscriptomic m6A Regulation of Axon Regeneration in the Adult Mammalian Nervous System. Neuron 2018, 97, 313–325.e6.
- Śledź, P.; Jinek, M. Structural insights into the molecular mechanism of the m6A writer complex. eLife 2016, 5, e18434.
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317.
- Schöller, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, localization, and phosphorylation of the m6A generating METTL3–METTL14–WTAP complex. RNA 2018, 24, 499–512.
- Ping, X.-L.; Sun, B.-F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.-J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.-S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189.
- Patil, D.P.; Chen, C.-K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373.
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887.
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29.
- Vitali, P.; Basyuk, E.; Le Meur, E.; Bertrand, E.; Muscatelli, F.; Cavaillé, J.; Huttenhofer, A. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J. Cell Biol. 2005, 169, 745–753.
- Purchal, M.K.; Eyler, D.E.; Tardu, M.; Franco, M.K.; Korn, M.M.; Khan, T.; McNassor, R.; Giles, R.; Lev, K.; Sharma, H.; et al. Pseudouridine synthase 7 is an opportunistic enzyme that binds and modifies substrates with diverse sequences and structures. Proc. Natl. Acad. Sci. USA 2022, 119, e2109708119.
- Akhtar, J.; Lugoboni, M.; Junion, G. m6A RNA modification in transcription regulation. Transcription 2021, 12, 266–276.
- Livneh, I.; Moshitch-Moshkovitz, S.; Amariglio, N.; Rechavi, G.; Dominissini, D. The m6A epitranscriptome: Transcriptome plasticity in brain development and function. Nat. Rev. Neurosci. 2020, 21, 36–51.
- Widagdo, J.; Wong, J.J.-L.; Anggono, V. The m6A-epitranscriptome in brain plasticity, learning and memory. Semin. Cell Dev. Biol. 2022, 125, 110–121.
- Chen, X.; Yu, C.; Guo, M.; Zheng, X.; Ali, S.; Huang, H.; Zhang, L.; Wang, S.; Huang, Y.; Qie, S.; et al. Down-Regulation of m6A mRNA Methylation Is Involved in Dopaminergic Neuronal Death. ACS Chem. Neurosci. 2019, 10, 2355–2363.
- Lin, L.; Hales, C.M.; Garber, K.; Jin, P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum. Mol. Genet. 2014, 23, 3299–3306.
- Sevgi, M.; Rigoux, L.; Kühn, A.B.; Mauer, J.; Schilbach, L.; Hess, M.E.; Gruendler, T.O.; Ullsperger, M.; Stephan, K.E.; Brüning, J.C.; et al. An Obesity-Predisposing Variant of the FTO Gene Regulates D2R-Dependent Reward Learning. J. Neurosci. 2015, 35, 12584–12592.
More
