Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Cosimo D. Altomare.
Polyphenolic compounds, encompassing flavonoids (e.g., quercetin, rutin, and cyanidin) and non-flavonoids (e.g., gallic acid, resveratrol, and curcumin), show several health-related beneficial effects, which include antioxidant, anti-inflammatory, hepatoprotective, antiviral, and anticarcinogenic properties, as well as the prevention of coronary heart diseases. Polyphenols have also been investigated for their counteraction against the adverse effects of common anticancer chemotherapeutics.
- polyphenols
- chemotherapy adjuvants
- oxidative stress
1. Introduction
Polyphenols constitute a major class of phytochemicals showing favorable effects on various pathologic conditions. They are plant-derived metabolites mainly originating from the acetate–malonate and shikimate biosynthetic pathways and they mostly exist as glycosides or are conjugated with other moieties (e.g., amines, carboxylic acids, lipids, and other phenols) [1,2][1][2]. Natural polyphenols include flavonoids and nonflavonoid compounds (e.g., phenolic acids and their esters, stilbenoids, and curcuminoids) [3].
Several studies have highlighted the relationships between dietary polyphenols and lower incidences of cancer, chronic heart diseases, and neurodegenerative syndromes [4,5,6,7][4][5][6][7]. The Mediterranean diet is associated with a reduced risk of cardiovascular disease, thanks to an adequate intake of olive oil, red wine, and anthocyanin-containing fruits and vegetables [8,9][8][9]. Other beneficial health effects, such as anti-inflammatory, antioxidant, antiallergic, antithrombotic, and antiviral activities, are related to dietary polyphenol intake [10,11,12][10][11][12]. Increasing lines of evidence have shown a relationship between some of the aforementioned diseases and oxidative stress resulting from the generation of reactive oxygen (ROS) and nitrogen species (RNS) [13], but no natural antioxidant has been approved so far for any therapeutic indication, except for the nutrient content claims for dietary supplements and conventional foods.
2. Protective Effects of Polyphenols against Adverse Effects of Antitumor Therapies
2.1. Polyphenolic Adjuvants in Anticancer Therapeutic Interventions
In a study conducted on MCF-7 cells (human breast cancer), ellagic acid was proven to (i) increase cell death, (ii) reduce cells’ capacity to form colonies, and (iii) accumulate cells in the sub-G1 (apoptotic) phase after gamma radiation treatment [50][14]. The effects were significantly higher for the combined treatment compared to the ellagic acid or irradiation treatment alone, thereby demonstrating the ability of ellagic acid to radio-sensitize MCF-7 cells. Interestingly, ellagic acid showed radio-protective effects on normal murine cell line in vitro. Fractions from wine extracts, mainly containing procyanidins, catechins, and flavonols, have shown an antiproliferative effect on PC3 cells (prostate cancer) in a dose-dependent manner [51][15]. These fractions induced autophagy on the same cell line, thus corroborating the potential to prevent the disease. In a recent in vitro study [52][16], ovarian cell lines were treated with oleuropein (a phenolic compound present in the fruits and leaves of olive trees). In particular, the authors showed that after using oleuropein to treat A2780 and A2780 cisplatin resistance cell lines, the expression of p21 and p53 increased, while the expression of Bcl-2 decreased. As a result, oleuropein was able to induce apoptosis, reduce cell proliferation, and reduce resistance to cisplatin in ovarian cell lines. Hydroxytyrosol, the product of oleuropein hydrolysis, is an effective anti-inflammatory and antioxidant polyphenol. It is able to reduce the nephrotoxicity from cisplatin by inhibiting chemokine-like factor 1 (CKLF1) involved in inflammation pathways and to induce anti-oxidative stress and anti-apoptosis activities in the kidneys of mice [53][17]. Honey contains a mixture of different active compounds (Figure 1), including coumaric acids (7–9), caffeic acid (10), ferulic acid (11), eugenol (12), and flavonoids, such as quercetin, apigenin, chrysin (13), pinocembrin (14), pinobanksin (15), and naringin (16) in different percentages depending on the floral source and geographical origins. An increasing amount of evidence has attributed a potential chemopreventive activity to honey [54][18]. In fact, multi-floral honey prevented the formation of breast cancer induced by 7,12-dimethylbenz(a)anthracene (DMBA) in a rat model [55][19]. Moreover, an increased level of bone marrow lymphocytes and peritoneal macrophages in mice suggested the activation of the immune system [56][20]. Oral mucositis (OM), which is one of the most common side effects of chemotherapy, could be reduced by honey, thanks to its capacity to increase the immune system response [57][21]. This effect was confirmed by a double-blind randomized clinical trial in which the patients affected by OM after chemotherapy were treated with betamethasone, honey, and a combination of honey and coffee [58][22].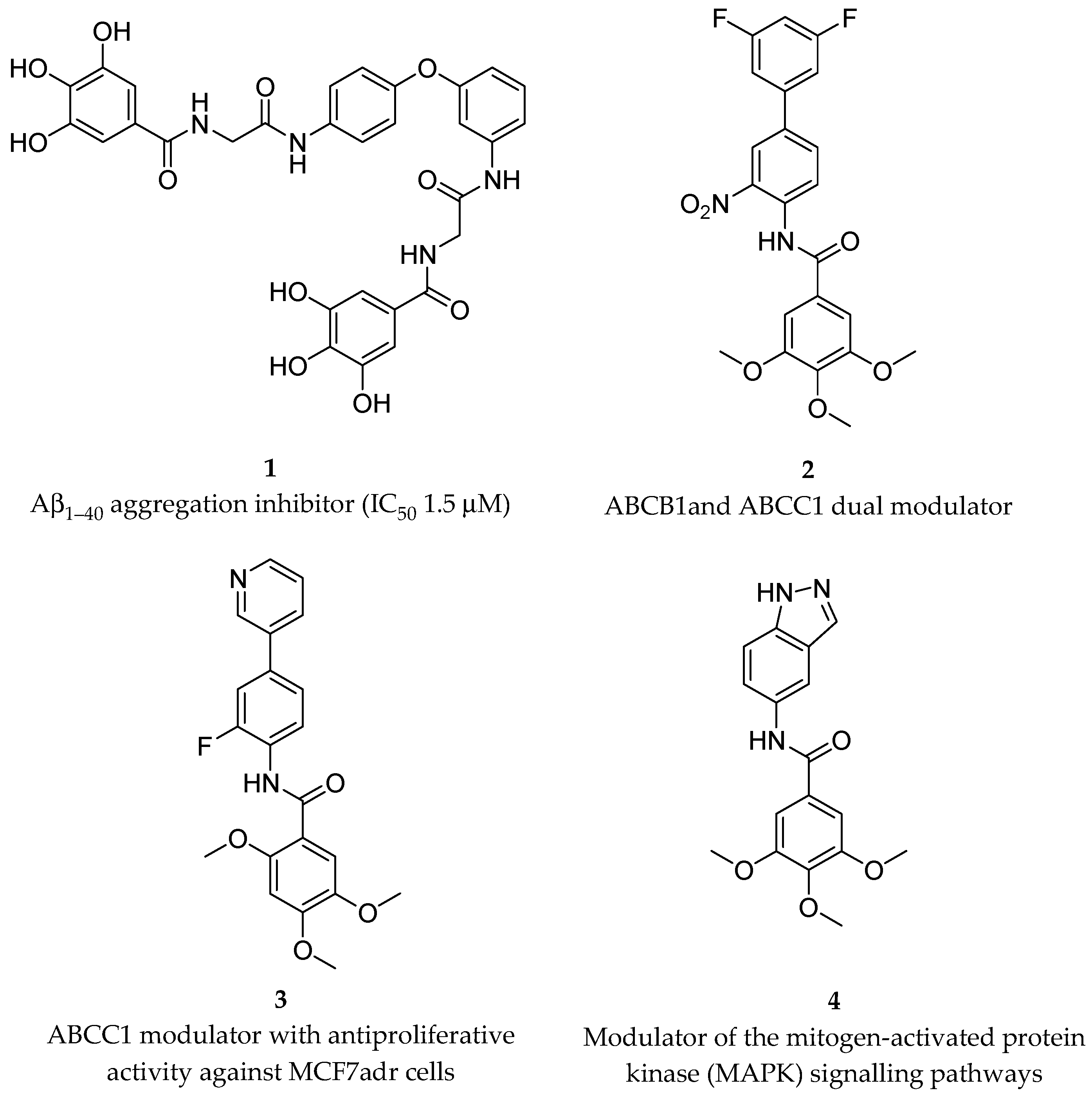
Figure 1. Structures of some polyphenolic components of honey.
2.2. Activity against ROS-Mediated Effects of Chemotherapeutics
Anthracyclines are anticancer antibiotics characterized by an anthraquinone moiety branched with an amino sugar at C-7. Doxorubicin (17) and daunorubicin (18) (Figure 2), isolated from the bacteria Streptomyces peucetius, were the earliest drugs of this family that entered clinical practice for cancer treatment [66][30]. Daunorubicin is effective in acute lymphocytic and myeloid leukemia, while doxorubicin is a component of polypharmacological protocols for treating solid tumors (e.g., breast cancer, soft tissue sarcomas, and aggressive lymphomas). Even though it is a common chemotherapeutic, the clinical use of doxorubicin is limited by its dose-dependent cardiac toxicity, which may lead to severe and irreversible forms of cardiomyopathy [67,68][31][32]. Indeed, anthracyclines can induce the early onset of progressive chronic cardiotoxicity, usually within one year of treatment [69][33]. Cardiomyopathy may persist or advance even after the discontinuation of therapy [70][34].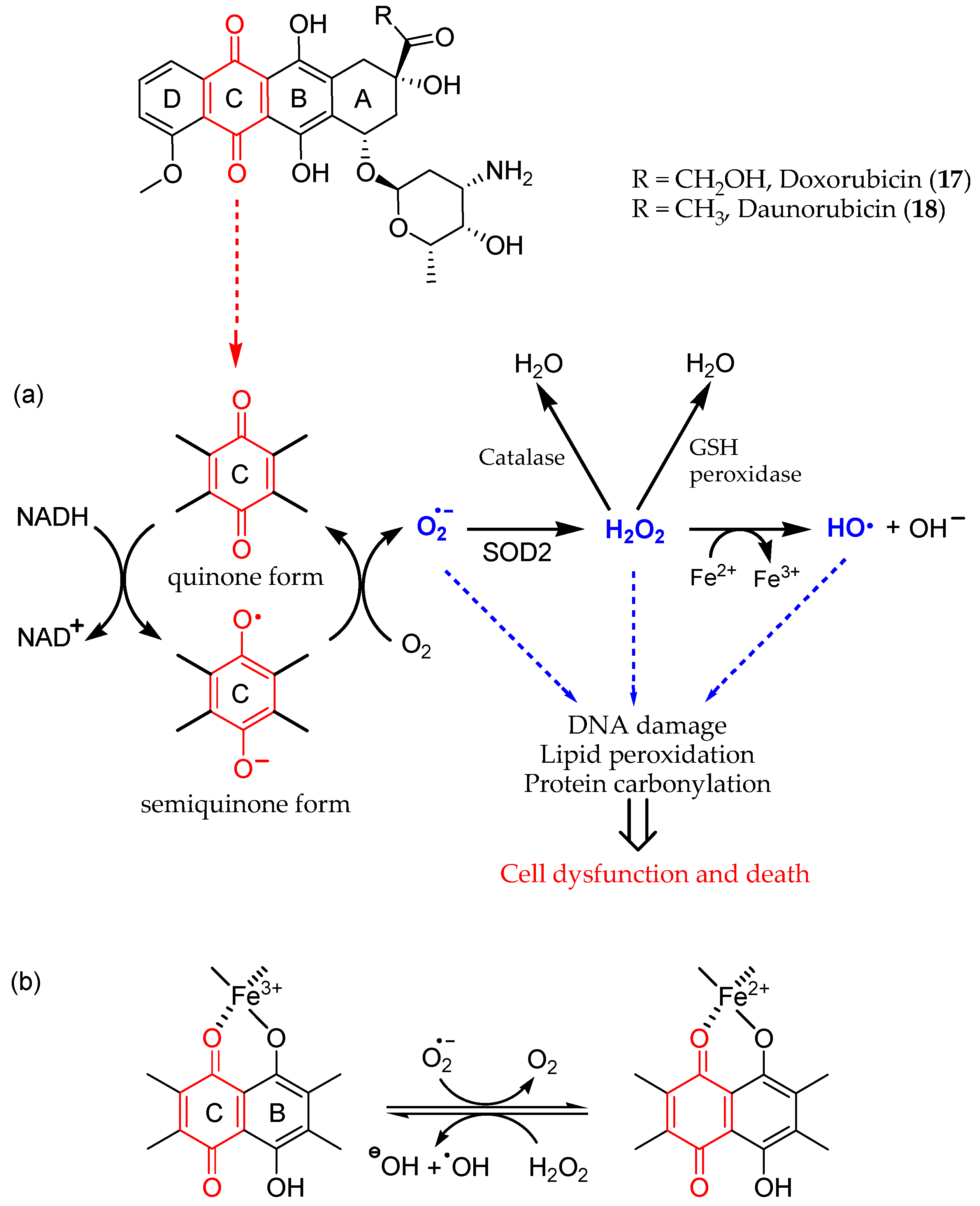
Figure 2. Redox cycling of anthracyclines 17 and 18, leading to ROS production and cardiotoxicity; it involves (a) the quinone C ring and (b) iron chelation (metal ion binding sites in B and C rings).
2.2.1. Preclinical Findings
Ellagic acid (19, Figure 3) is a product of the hydrolysis of ellagitannins. Recent pharmacological studies have demonstrated that 19 acts as a free-radical scavenger, with several health benefits, such as anti-inflammatory, antihepatotoxic, antisteatosic, anticholestatic, antifibrogenic, antidiabetic, hypolipidemic, and antiatherosclerotic effects [76,77][40][41]. Moreover, ellagic acid has been proven to inhibit type-B monoamine oxidase (MAO-B) [78][42], thereby protecting rat brains from 6-hydroxydopamine-induced neuroinflammation in a model of Parkinson’s disease [79][43] and preventing scopolamine- and diazepam-induced cognitive impairments [80][44]. In male Wistar rats, orally administered 19 was proven to attenuate the doxorubicin-induced oxidative process in myocardial tissue [81][45].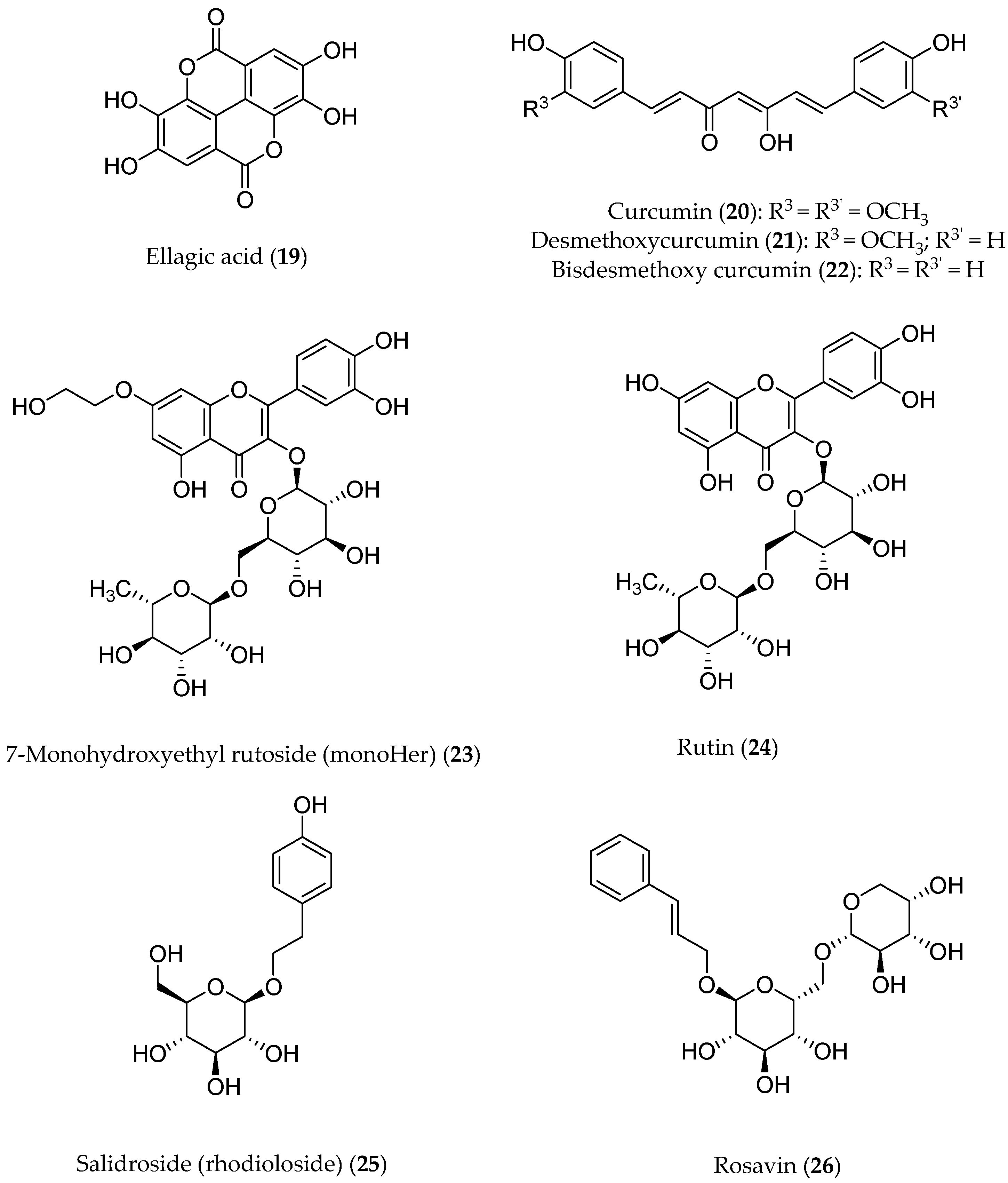
Figure 3. Structures of polyphenols that have been pharmacologically investigated.
2.2.2. Evaluation of Clinical Studies
The flavonoid 7-mono-O-(β-hydroxyethyl)rutoside (monoHER) (23, Figure 3), a semi-synthetic derivative of rutin (24) bearing rutinose (α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranose) as the disaccharide moiety, has been shown to protect mice against doxorubicin-induced cardiotoxicity without adverse effects at a very high dose (500 mg/kg) [91][55]. Based on these results, clinical trials were performed to evaluate its protective effects in cancer patients. MonoHER, administered intravenously at a 1500 mg/m2 dose 60 min before doxorubicin was administered, was evaluated through an endomyocardial biopsy, but the benefits observed in these preclinical studies were not confirmed [92][56]. These conflicting results might be attributable to interspecies differences in ADME (absorption, distribution, metabolism, and excretion). However, the antitumor activity of doxorubicin appeared to greatly improve, even displaying a partial remission of metastatic soft-tissue sarcoma in some patients. These results are somehow in agreement with the potentiating anti-proliferative effects observed in vitro for a number of flavonoids [93][57]. Another noteworthy clinically investigated polyphenol is salidroside (i.e., tyrosol glucoside, 25) found in Rhodiola rosea and used in traditional Tibetan medicine. Salidroside (Figure 3), along with the less active rosavin 26 (a cinnamyl alcohol glycoside bearing α-l-arabinopyranosyl-α-d-glucopyranoside as the disaccharide moiety), was reported to play a role in reducing mitochondrial-generated ROS and apoptosis signaling [94][58]. Pretreatment with salidroside appears to significantly reduce in vitro both ROS and mitochondrial superoxide overproduction [95][59] as well as to arrest the cell cycle and apoptosis in human breast cancer cells [96][60]. Furthermore, 25 showed antioxidant-related cardiovascular protection [97][61]. These results led to clinical studies to assess its effectiveness in protecting against cardiac dysfunctions induced by epirubicin in sixty patients with histologically confirmed breast cancer. In this trial, all the patients had a scheduled cumulative epirubicin dose of 400 mg/m2. Although the oral co-administration of 25 and epirubicin was well tolerated in all of the patients, no significant differences in the protection from epirubicin-induced cardiotoxic effects were found compared to the placebo groups, once again suggesting that most likely the poor bioavailability of the polyphenolic phytochemicals in humans is a major factor limiting its clinical application [98][62].2.3. Bioavailability Issues
A main hurdle to the therapeutic application of polyphenols is their poor bioavailability. Despite their antioxidant, antiphlogistic, and anticancer pharmacological activities, which may synergistically cooperate in antitumor chemotherapy, polyphenols show poor bioavailability, which strongly limits their efficacy. Several factors, such as low solubility/permeability, photochemical isomerization, auto-oxidation, and hepatic/intestinal rapid metabolic processes, just to name the main ones, negatively affect their bioavailability and in fact represent major obstacles to their therapeutic use. Nanodelivery systems may have the potential to improve their therapeutic efficacy. While the reader may refer to recent, more exhaustive reviews on these topics [4[4][10],10], herein wresearchers would like to draw attention to some promising applications in CNS diseases [46][63] or in cancer prevention and therapy [99][64]. For example, numerous studies have proven that curcumin (20) is chemically and metabolically unstable and thereby poorly bioavailable [88][52]. A spectroscopic analysis revealed that a major degradation product (27) is formed by the autoxidation of 20 [100][65], whereas three minor degradation products, namely vanillin (28), ferulic acid (29), and the ketone product 30, are generated via a solvolysis reaction in an aqueous alkaline buffer [101][66] (Figure 4). Pharmaceutical nanotechnologies may implement efficient delivery systems aimed at improving the bioavailability of polyphenols [102][67].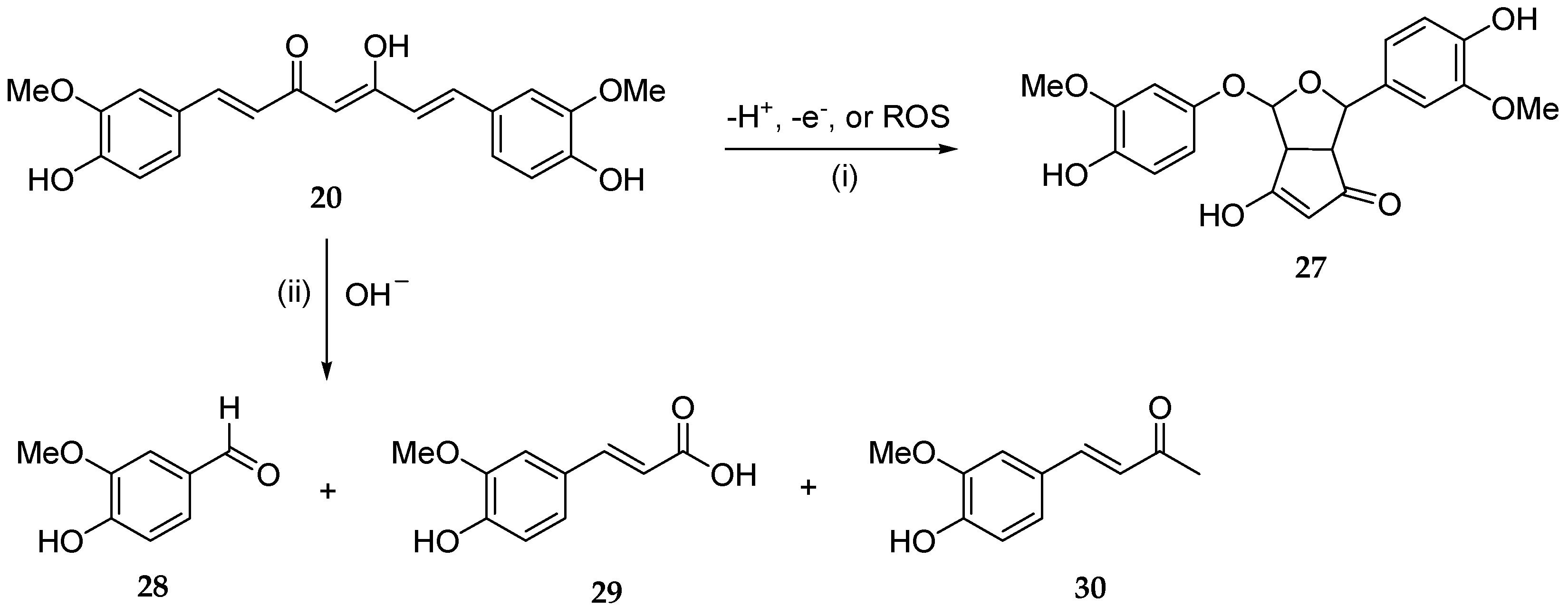
Figure 4. Main chemical degradation reactions of curcumin (20): (i) autoxidation in aqueous buffered medium; (ii) solvolysis under alkaline pH in aqueous buffer.
References
- Nan Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, 3248S–3254S.
- Feng, C.; Chen, B.; Fan, R.; Zou, B.; Han, B.; Guo, G. Polyphenol-Based Nanosystems for Next-Generation Cancer Therapy: Multifunctionality, Design, and Challenges. Macromol. Biosci. 2023, 23, 2300167.
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary Phytochemicals and Cancer Chemoprevention: A Review of the Clinical Evidence. Oncotarget 2016, 7, 52517–52529.
- Almeida, S.; Alves, M.G.; Sousa, M.; Oliveira, P.F.; Silva, B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016, 30, 345–366.
- Hurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827.
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; De Gaetano, G.; on behalf of the MOLI-SANI Study Investigators. Mediterranean Diet, Dietary Polyphenols and Low Grade Inflammation: Results from the MOLI-SANI Study: Mediterranean Diet, Polyphenols and Low Grade Inflammation. Br. J. Clin. Pharmacol. 2017, 83, 107–113.
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-Rich Foods in the Mediterranean Diet Are Associated with Better Cognitive Function in Elderly Subjects at High Cardiovascular Risk. JAD 2012, 29, 773–782.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential Use of Dietary Natural Products, Especially Polyphenols, for Improving Type-1 Allergic Symptoms. CPD 2014, 20, 857–863.
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A Review of the Mechanisms and Effectiveness of Dietary Polyphenols in Reducing Oxidative Stress and Thrombotic Risk. J. Hum. Nutr. Diet. 2014, 27, 1–21.
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The Oxidative Stress Theory of Disease: Levels of Evidence and Epistemological Aspects: The Oxidative Stress Theory of Disease. Br. J. Pharmacol. 2017, 174, 1784–1796.
- Ahire, V.; Kumar, A.; Mishra, K.P.; Kulkarni, G. Ellagic Acid Enhances Apoptotic Sensitivity of Breast Cancer Cells to γ-Radiation. Nutr. Cancer 2017, 69, 904–910.
- Tenta, R.; Fragopoulou, E.; Tsoukala, M.; Xanthopoulou, M.; Skyrianou, M.; Pratsinis, H.; Kletsas, D. Antiproliferative Effects of Red and White Wine Extracts in PC-3 Prostate Cancer Cells. Nutr. Cancer 2017, 69, 952–961.
- Hashemi Sheikhshabani, S.; Amini-Farsani, Z.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asgharzade, S. Oleuropein Reduces Cisplatin Resistance in Ovarian Cancer by Targeting Apoptotic Pathway Regulators. Life Sci. 2021, 278, 119525.
- Chen, C.; Ai, Q.; Wei, Y. Hydroxytyrosol Protects against Cisplatin-Induced Nephrotoxicity via Attenuating CKLF1 Mediated Inflammation, and Inhibiting Oxidative Stress and Apoptosis. Int. Immunopharmacol. 2021, 96, 107805.
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the Hive: Honey, a Novel Weapon against Cancer. Eur. J. Med. Chem. 2017, 142, 290–299.
- Takruri, H.R.; Shomaf, M.S.; Shnaigat, S.F. Multi Floral Honey Has a Protective Effect against Mammary Cancer Induced by 7,12-Dimethylbenz(a)Anthracene in Sprague Dawley Rats. JAS 2017, 9, 196.
- Attia, W.Y.; Gabry, M.S.; El-Shaikh, K.A.; Othman, G.A. The Anti-Tumor Effect of Bee Honey in Ehrlich Ascite Tumor Model of Mice is Coincided with Stimulation of the Immune Cells. EJI 2008, 15, 169–183.
- Lalla, R.V.; Brennan, M.M.; Schubert, M.M. Oral complications of cancer therapy. In Pharmacology and Therapeutics for Dentistry, 6th ed.; Yagiela, J.A., Dowd, F.J., Johnson, B., Mariotti, A., Neidle, E.A., Eds.; Mosby Elsevier: Amsterdam, The Netherlands, 2011; pp. 782–798.
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus Honey” versus “Topical Steroid” in the Treatment of Chemotherapy-Induced Oral Mucositis: A Randomised Controlled Trial. BMC Complement. Altern. Med. 2014, 14, 293.
- Charalambous, A.; Lambrinou, E.; Katodritis, N.; Vomvas, D.; Raftopoulos, V.; Georgiou, M.; Paikousis, L.; Charalambous, M. The Effectiveness of Thyme Honey for the Management of Treatment-Induced Xerostomia in Head and Neck Cancer Patients: A Feasibility Randomized Control Trial. Eur. J. Oncol. Nur 2017, 27, 1–8.
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey Protects against Cisplatin-Induced Hepatic and Renal Toxicity through Inhibition of NF-κB-Mediated COX-2 Expression and the Oxidative Stress Dependent BAX/Bcl-2/Caspase-3 Apoptotic Pathway. Food Funct. 2018, 9, 3743–3754.
- Study Details | Polyphenol Rich Aerosol as a Support for Cancer Patients in Minimizing Side Effects After a Radiation Therapy—NCT05994638| ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05994638 (accessed on 11 November 2023).
- Study Details | Investigation of a Polyphenol-Rich Preparation as Support for Oncology Patients Undergoing Gastrointestinal Tumor Resection—NCT06017661 | ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06017661 (accessed on 4 November 2023).
- Xue, D.; Peng, Y.; Zhang, M.; Zheng, L.; Liang, Q.; Li, H.; Yu, J.S.; Chen, J.T. Compositions and Methods for Preventing and Treating Radiation-Induced Bystander Effects Caused by Radiation or Radio-Therapy—CN111447940. 2020. Available online: https://worldwide.espacenet.com/patent/search/family/065015329/publication/CN111447940A?q=CN111447940 (accessed on 29 October 2023).
- Study Details | Pilot Study of a MIND Diet Intervention in Women Undergoing Active Treatment for Breast Cancer—NCT05984888| ClinicalTrials.Gov. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05984888 (accessed on 10 November 2023).
- Study Details | Supplementation with Dietary Anthocyanins and Side Effects of Radiotherapy for Breast Cancer—NCT02195960| ClinicalTrials.Gov. 2023. Available online: https://clinicaltrials.gov/study/NCT02195960 (accessed on 28 October 2023).
- Fujiwara, A.; Hoshino, T.; Westley, J.W. Anthracycline Antibiotics. Crit. Rev. Biotechnol. 1985, 3, 133–157.
- Brown, S.-A.; Sandhu, N.; Herrmann, J. Systems Biology Approaches to Adverse Drug Effects: The Example of Cardio-Oncology. Nat. Rev. Clin. Oncol. 2015, 12, 718–731.
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation 2015, 131, 1981–1988.
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. CCR 2012, 7, 214–220.
- Vejpongsa, P.; Yeh, E.T.H. Prevention of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2014, 64, 938–945.
- Jungsuadee, P. Doxorubicin-Induced Cardiomyopathy: An Update beyond Oxidative Stress and Myocardial Cell Death. Cardiovasc. Regen. Med. 2016, 3, e1127.
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J. Clin. Invest. 2014, 124, 617–630.
- Gammella, E.; Maccarinelli, F.; Buratti, P.; Recalcati, S.; Cairo, G. The Role of Iron in Anthracycline Cardiotoxicity. Front. Pharmacol. 2014, 5, 25.
- Schuler, M.K.; Gerdes, S.; West, A.; Richter, S.; Busemann, C.; Hentschel, L.; Lenz, F.; Kopp, H.-G.; Ehninger, G.; Reichardt, P.; et al. Efficacy and Safety of Dexrazoxane (DRZ) in Sarcoma Patients Receiving High Cumulative Doses of Anthracycline Therapy—A Retrospective Study Including 32 Patients. BMC Cancer 2016, 16, 619.
- Lehenbauer Ludke, A.R.; Al-Shudiefat, A.A.-R.S.; Dhingra, S.; Jassal, D.S.; Singal, P.K. A Concise Description of Cardioprotective Strategies in Doxorubicin-Induced cardiotoxicityThis Article Is One of a Selection of Papers Published in a Special Issue Celebrating the 125th Anniversary of the Faculty of Medicine at the University of Manitoba. Can. J. Physiol. Pharmacol. 2009, 87, 756–763.
- De Oliveira, M.R. The Effects of Ellagic Acid upon Brain Cells: A Mechanistic View and Future Directions. Neurochem. Res. 2016, 41, 1219–1228.
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, Ellagic Acid and Vascular Health. Mol. Aspects Med. 2010, 31, 513–539.
- Khatri, D.; Juvekar, A. Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Phcog Mag. 2016, 12, 116.
- Farbood, Y.; Sarkaki, A.; Dolatshahi, M.; Mansouri, S.M.T.; Khodadadi, A. Ellagic Acid Protects the Brain Against 6-Hydroxydopamine Induced Neuroinflammation in a Rat Model of Parkinson’s Disease. Basic.Clinic. Neurosci. 2015, 6, 83–89.
- Mansouri, M.T.; Farbood, Y.; Naghizadeh, B.; Shabani, S.; Mirshekar, M.A.; Sarkaki, A. Beneficial Effects of Ellagic Acid against Animal Models of Scopolamine- and Diazepam-Induced Cognitive Impairments. Pharm. Biol. 2016, 54, 1947–1953.
- Warpe, V.S.; Mali, V.R.; S, A.; Bodhankar, S.L.; Mahadik, K.R. Cardioprotective Effect of Ellagic Acid on Doxorubicin Induced Cardiotoxicity in Wistar Rats. JACME 2015, 5, 1–8.
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal Importance of Gallic Acid and Its Ester Derivatives: A Patent Review. Pharm. Pat. Anal. 2015, 4, 305–315.
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid. and Related Compounds as Neuroprotective Agents: You Are What You Eat! CPB 2014, 15, 362–372.
- Kulkarni, J.; Swamy, A.H.M.V. Cardioprotective Effect of Gallic Acid against Doxorubicin-Induced Myocardial Toxicity in Albino Rats. Indian. J. Health Sci. Biomed. Res. 2015, 8, 28.
- Pothitirat, W.; Gritsanapan, W. Variation of Bioactive Components in Curcuma Longa in Thailand. Curr. Sci. 2006, 91, 1397–1400.
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular Mechanisms Underlying Chemopreventive Potential of Curcumin: Current Challenges and Future Perspectives. Life Sci. 2016, 148, 313–328.
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases: Curcumin: From Kitchen to Clinic. Br. J. Pharmacol. 2017, 174, 1325–1348.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637.
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218.
- El-Sayed, E.M.; El-azeem, A.S.A.; Afify, A.A.; Shabana, M.H.; Ahmed, H.H. Cardioprotective Effects of Curcuma Longa, L. Extracts against Doxorubicin-Induced Cardiotoxicity in Rats. J. Med. Plants Res. 2011, 5, 4049–4058.
- Van Acker, F.A.A.; Van Acker, S.A.B.E.; Krammer, K.; Haenen, G.R.M.M.; Bast, A.; Van Der Vijgh, W.J.F. 7-Monohydroxyethylrutoside Protects against Chronic Doxorubicin-Induced Cardiotoxicity When Administered Only Once per Week. Clin. Cancer Res. 2000, 6, 1337–1341.
- Bruynzeel, A.M.E.; Niessen, H.W.M.; Bronzwaer, J.G.F.; Van Der Hoeven, J.J.M.; Berkhof, J.; Bast, A.; Van Der Vijgh, W.J.F.; Van Groeningen, C.J. The Effect of Monohydroxyethylrutoside on Doxorubicin-Induced Cardiotoxicity in Patients Treated for Metastatic Cancer in a Phase II Study. Br. J. Cancer 2007, 97, 1084–1089.
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids Inhibit Cell Proliferation and Induce Apoptosis and Autophagy through Downregulation of PI3Kγ Mediated PI3K/AKT/mTOR/p70S6K/ULK Signaling Pathway in Human Breast Cancer Cells. Sci. Rep. 2018, 8, 11255.
- Zhong, H.; Xin, H.; Wu, L.-X.; Zhu, Y.-Z. Salidroside Attenuates Apoptosis in Ischemic Cardiomyocytes: A Mechanism Through a Mitochondria-Dependent Pathway. J. Pharmacol. Sci. 2010, 114, 399–408.
- Schriner, S.E.; Abrahamyan, A.; Avanessian, A.; Bussel, I.; Maler, S.; Gazarian, M.; Holmbeck, M.A.; Jafari, M. Decreased Mitochondrial Superoxide Levels and Enhanced Protection against Paraquat in Drosophila Melanogaster Supplemented with Rhodiola Rosea. Free. Radic. Res. 2009, 43, 836–843.
- Hu, X.; Zhang, X.; Qiu, S.; Yu, D.; Lin, S. Salidroside Induces Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cells. BBRC 2010, 398, 62–67.
- Wu, T.; Zhou, H.; Jin, Z.; Bi, S.; Yang, X.; Yi, D.; Liu, W. Cardioprotection of Salidroside from Ischemia/Reperfusion Injury by Increasing N-Acetylglucosamine Linkage to Cellular Proteins. Eur. J. Pharmacol. 2009, 613, 93–99.
- Zhang, H.; Shen, W.; Gao, C.; Deng, L.; Shen, D. Protective Effects of Salidroside on Epirubicin-Induced Early Left Ventricular Regional Systolic Dysfunction in Patients with Breast Cancer. Drugs R. D. 2012, 12, 101–106.
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of Dietary Polyphenols: Factors Contributing to Their Clinical Application in CNS Diseases. Neurochem. Int. 2015, 89, 198–208.
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol Nanoformulation for Cancer Prevention and Therapy: Resveratrol Nanoformulations for Cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31.
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and Cyclooxygenase-2 Catalyzed Transformation of the Dietary Chemopreventive Agent Curcumin. J. Biol. Chem. 2011, 286, 1114–1124.
- Gordon, O.N.; Schneider, C. Vanillin and Ferulic Acid: Not the Major Degradation Products of Curcumin. Trends Mol. Med. 2012, 18, 361–363.
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric Nanoparticles Encapsulating White Tea Extract for Nutraceutical Application. J. Agric. Food Chem. 2015, 63, 2026–2032.
More
