This review discusses, iIn-depth, the role of dopamine in locomotor function is discussed in aging and Parkinson's disease. It highlights the inherent differences in dopamine regulation within the nigrostriatal neuron in striatum as compared to substantia nigra. Given the copious amount of evidence of differential dopamine regulation between striatum and substantia nigra, the review builds the case that dopamine signaling in the substantia nigra is as equally important, if not more so, in regulation of locomotor function. The ever increasing evidence that nigral dopamine can regulate locomotor function has recently come to light in the past 10 years, reigniting the efforts that began over 30 years ago since the first studies that reported such a role.
- dopamine
- tyrosine hydroxylase
- dopamine receptor
- nigrostriatal
- Parkinson’s disease
1. Introduction
2. Insights of How Striatal DA Signaling Affects Locomotor Function Have Reached a Plateau
In the context of PD, DA is, by far, the most studied of the catecholamines, with NE running a distant second. Since 1962, there have been ~29,000 publications associated with DA and PD vs. ~1700 associated with NE and PD. The evidence for deficient nigrostriatal DA signaling as the primary cause of motor symptoms of PD is strong. Yet there still remains a critical unresolved issue that hampers progress: a continuous perseverating focus to attribute deficient DA signaling in the striatum as the sole culprit for motor impairment. This focus is undoubtedly driven by the longstanding working model of basal ganglia circuit dysfunction that arises from the loss of striatal DA due to the progressive loss of nigrostriatal neurons. It is argued that this striato-centric focus has generated a plateau in our understanding of exactly how any of the five steps of neurotransmission with deficient DA-regulating function in striatum actually impair motor function. For definition purposes, the relation of nigrostriatal DA signaling to motor impairment will focus upon bradykinesia/hypokinesia, which is among four cardinal signs of PD which also include rigidity and postural instability and tremor at rest. Indeed, there are clinically based examples of where improvements in striatal DA signaling did not equate to alleviation of motor impairment in PD patients [60][64][65]. More evidence of this lack of alignment between striatal DA levels and severity of motor impairment is seen at the later stages of PD. Although the severity of motor impairment continues to worsen 4 to 5 years after PD diagnosis, loss of striatal DA-regulating proteins or signaling has already reached near 100% [20][66][67][68][69]. There is a comparable amount of evidence for this misalignment between striatal DA levels and motor function status in pre-clinical studies of rat PD models [22][57][58][59][70][71][72][73][74][75][76]. Motor impairment may also be present with far less than 80%, if any, striatal DA loss [54][65][70][72] or, conversely, motor impairment may not be present even though striatal DA loss meets or exceeds 80% [22][73][74]. Motor impairment can also be alleviated without any increase in or recovery of striatal DA or DA-regulating protein loss [54][57][59][73][74][75][76]. The weight of evidence that shows the influence of striatal DA signaling on basal ganglia circuits is too great to list here. However, the incongruities between the level of locomotor function and DA signaling in striatum can no longer be ignored if we are to solve which critical dopaminergic element(s) are to be targeted to maximize effective therapeutic strategies. The key question is where in the nigrostriatal pathway does DA have the greatest influence on locomotor function; particularly regarding the mechanisms that drive the initiation of self-generated movement. Although the evidence that nigral DA signaling can influence motor function is sparse, it has nonetheless been in existence since the 1980s [77][78][79][80][81]. The paucity of studies evaluating the SN is likely due to a prevailing presumption that neurotransmitter functions at the axon terminal are the sole influence of behavioral outcomes. Thus, interrogation of nigral DA signaling has not been considered in experimental designs to define how components of nigrostriatal DA signaling affect locomotor activity. In this light, it is reasonable to presume that the numerous ambiguities between striatal DA regulation and motor function that have accumulated in the literature over the past several decades could have been resolved if assessment of nigral DA signaling was included in the study design.3. Dissecting the Impact of the Five Components of DA Neurotransmission on Locomotor Function
As goes with the loss of nigrostriatal neurons in PD, the loss of DA-regulating proteins and processes involved in neurotransmission follows. Interference with the functions of any of these proteins or processes can also affect locomotor function in naïve (non-PD) animal models. Tyrosine hydroxylase (TH) is the rate-limiting step of DA biosynthesis, converting tyrosine to L-dihydroxyphenylalanine (L-DOPA). Inhibition of TH with alpha-methyl-p-tyrosine (AMPT) decreases DA tissue levels and inhibits locomotor activity [7][82][83][84][85][86]. In humans, inhibition of hyperkinetic movements, such as chorea, dystonia, or dyskinesia, can also be produced by AMPT [87][88]. The storage of DA and NE is controlled by vesicular monoamine transporter 2 (VMAT2), which imports monoamines like DA into synaptic vesicles using a proton gradient. This function is inhibited by reserpine, which also inhibits locomotor activity [89][90][91], as first identified by a parkinsonian symptom side effect produced in hypertension treatment [92]. VMAT2 is expressed in both striatum and SN [93][94], which confers the capacity for storing DA for eventual release in the entire nigrostriatal pathway. Once DA is packaged in synaptic vesicles, it can be released by neuronal activity or by modulation of transporter function through stimulant action. At the extracellular level, DA release from the nigrostriatal pathway is the step that delivers tissue content, via vesicular delivery, to the synapse [95][96][97][98], wherein DA has four fates, binding to the pre- or post-synaptic DA receptors, reuptake into the neuron, or diffusion away from the release site [99]. Drugs that target DA receptors, the post-synaptic DA D1 receptor or pre- and post-synaptic DA D2 receptor, also influence locomotor activity and are targets for pharmacotherapy in PD treatment [100]. An acute regimen of antipsychotics such as haloperidol or either DA D1 or D2 receptor antagonists reduce locomotor activity [101][102][103][104][105]. Conversely, DA D1 or D2 agonists increase locomotor activity in rodents and primates [43][106][107] and improve motor functions in late-stage human PD [108][109][110]. The release of DA can also be modulated by DA D2 autoreceptor function [111] in both striatum and SN [31][112]. Finally, it should be mentioned that although the focus of this research on DA receptors is upon the D1 receptor, with brief overview of the D2 receptor, the three other DA receptors have been recently shown to play a role in locomotor impairments of PD, particularly the D3 and D5 receptors [113][114][115][116]. Functionally, the regulation of DA release by neuronal activity is critical for initiation of locomotor activity [117][118][119][120][121]. Deficits in DA release, such as occurs in aging or from over-expression of alpha-synuclein, are associated with decreased locomotor activity [122][123][124]. Conversely, under conditions that increase DA release, such as induced by amphetamine or methamphetamine [125][126][127], there is increased locomotor activity [128][129][130]. The termination of DA signaling occurs by reduction of extracellular DA levels in the synapse, largely, though not exclusively [99], through reuptake by the dopamine transporter (DAT) [131][132][133]; a process that occurs in SN as well as striatum [134][135][136]. DAT protein expression is considerably greater in the striatum [94], and, not withstanding possible influences of trafficking or contributions of other monoamine transporters, this difference may explain why DA release and uptake dynamics differ between these two regions [134][135][136]. Through constant trafficking between cytosol and plasma membrane, DAT function is dynamically regulated, including aging and in PD [137][138][139]. The DAT, like the DA D2 receptor, also has considerable interaction with other components of DA neurotransmission, including DA D2 receptors [34][112], and has considerable influence on maintaining DA tissue levels, TH expression, and phosphorylation selectively in the striatum, but not in SN [140][141]. There is also evidence of plasticity in DA uptake under conditions where DA and DAT levels are particularly low. In such cases, the NE transporter may also transport DA, with inherently low DA innervation or from severe loss of nigrostriatal neuron terminals [142][143]. Given the considerable influence of DAT on DA homeostasis, locomotor activity is strongly affected by DAT expression levels. DAT knockout mice or rats show a hyperkinetic phenotype [144][145][146]. This hyperkinetic phenotype is not likely explained by the low DA uptake capacity in the striatum due to DAT knockout, as DA tissue content levels are severely reduced to a level that is comparable to nigrostriatal lesion (>90% loss) [140][141]. Systemic delivery of nomifensine, a DAT inhibitor, increases locomotor activity [147], consistent with the hyperkinetic phenotype of the knockout [144][145][146]. While presumably this effect would be considered to be due to elevated extracellular DA levels in striatum from interference with DA uptake, the researchers recently reported that infusion of nomifensine in striatum did not increase locomotor activity in aged rats, despite a striatum-specific increase in extracellular DA levels produced by nomifensine infusion therein [148].4. Nigrostriatal DA Signaling and PD-Related Motor Impairment
From the perspective of deficient DA signaling impact on motor impairment in PD, a long-standing unresolved issue is why motor impairment does not occur until there is 70–80% TH or DA loss in striatum. It was long thought that increased DA turnover reflected increased DA signaling during progressive loss of the nigrostriatal neuron terminals [19][73][149][150][151][152], thus compensating for TH protein loss to enable normal locomotor activity. L-DOPA, the product of TH, remains the gold standard for treating motor symptoms. Thus, it stands to reason that compensating for TH loss through engagement of innate compensatory mechanisms that increase DA levels would promote maintaining locomotor function until striatal TH loss was too severe. Increased DA turnover was proposed to be an indicator of enhanced DA signaling to compensate for TH protein loss during nigrostriatal neuron loss [19][52][73][149][150][151][152]. However, Bezard and colleagues definitively showed in an elegant timeline study using MPTP-lesioned primates in which increased DA turnover occurred only after bradykinesia manifested; there was no evidence of increased DA turnover during the asymptomatic period [19]. Also, 80% TH and DA loss in striatum appeared to be necessary for the onset of bradykinesia; even 60% TH loss in striatum was observed during the asymptomatic period. Fortunately, this study also assessed TH loss in the SN and found that, at the onset of motor impairment, there was ~40% loss in the SN; far less than 80% loss seen at the axon terminals. This loss in the SN may be related to regionally selective loss of nigral neurons, as shown in human aging and PD [153][154]. Also, this disparity in TH loss between SN and striatum has strong translational relevance because this disparity consistently manifests in human PD [20][66][67][68]. Nonetheless, the lack of evidence to support a role for increased DA turnover in striatum to offset the onset of locomotor impairment gave rise to the consideration that non-DA related mechanisms to be responsible for delaying the onset of motor impairment [52]. Recent work from the researchers' group indicates that the compensatory mechanism to mitigate the severity of hypokinesia and delay its onset against progressive nigrostriatal neuron loss is related to increased DA signaling in the SN, and not striatum [22]. This mechanism involves an increase in ser31 TH phosphorylation, specifically in the SN, that begins early after nigrostriatal loss induction by 6-hydroxydopamine (6-OHDA) and is maintained at least until neuronal loss reaches 80% in the SN. As a result of this increase in ser31 TH phosphorylation, there is less loss of DA as compared to TH throughout neuronal loss [22]. This differential in DA and TH loss also manifests in the SN, contralateral to the lesioned side, as TH loss begins there at a later time after lesion induction. When correlating the loss of DA in SN and striatum against the severity of motor decline in the open field, only DA loss in the SN has significant correlation [22]. In striatum, the researchers found no difference in TH and DA loss, as both exceeded 90% early after lesion induction, commensurate with decreased ser31 TH phosphorylation and increased DA turnover. In contrast, DA turnover decreased in the SN as neuron loss progressed. The findings of diminished lesion impact on DA tissue content in the SN are also reflected in the extracellular realm, wherein baseline DA levels are unaffected by 6-OHDA lesions despite severe neuronal loss [155]. Together, these results frame a new perspective on the mechanism by which motor impairment is delayed by increased DA biosynthesis in the SN, despite progressive nigrostriatal neuron loss that occurs in PD (Figure 1). Moreover, these results are disease-relevant and further support a role for nigral DA signaling in locomotor function.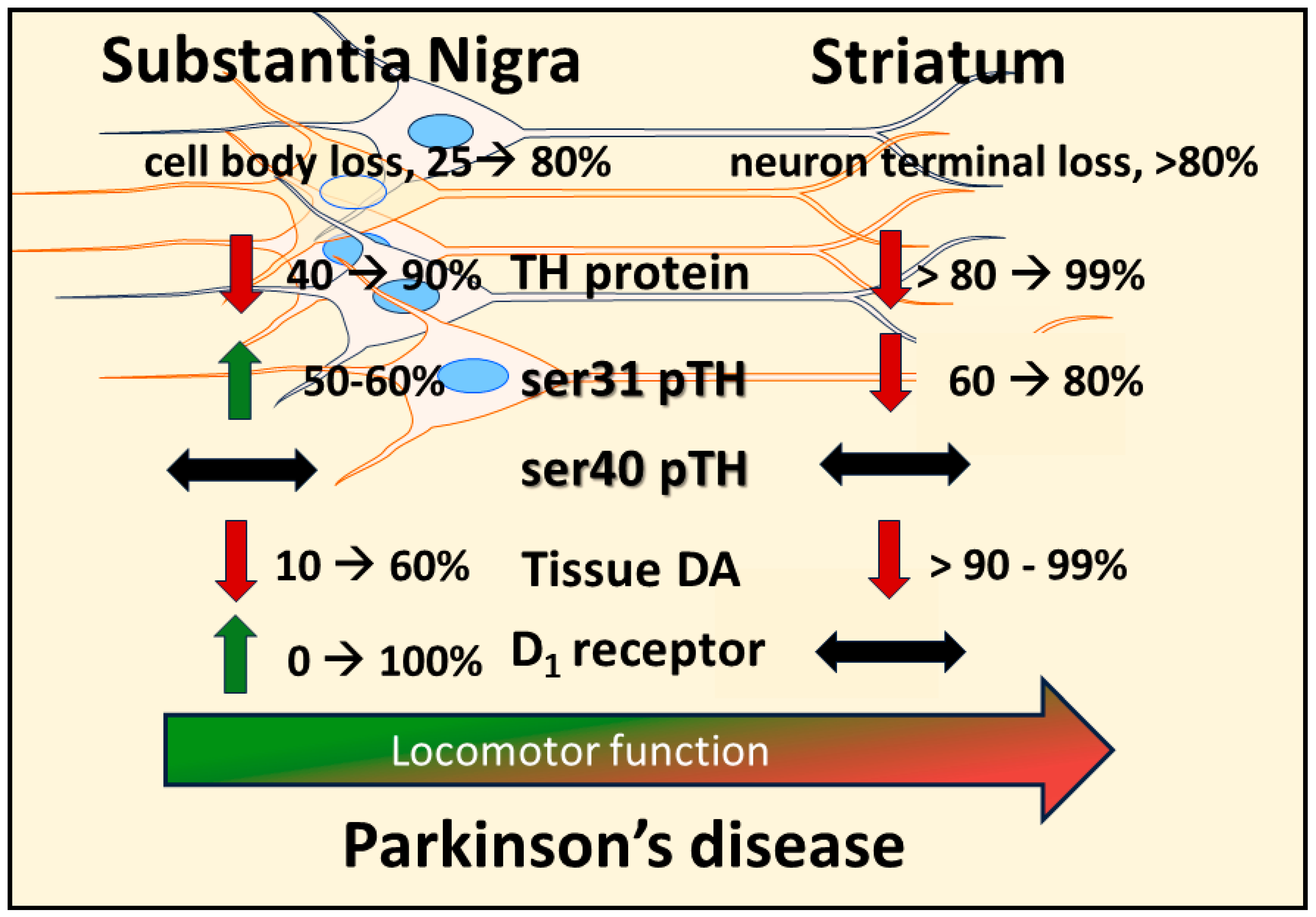
References
- Glowinski, J.; Axelrod, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain. IV. Effects of drugs on the disposition and metabolism of H3-norepinephrine and H3-dopamine. J. Pharmacol. Exp. Ther. 1966, 153, 30–41.
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain. I. The disposition of norepinephrine, dopamine and dopa in various regions of the brain. J. Neurochem. 1966, 13, 655–669.
- Axelrod, J. Noradrenaline: Fate and Control of its biosynthesis. Science 1971, 173, 598–606.
- Thierry, A.M.; Blanc, G.; Sobel, A.; Stinus, L.; Glowinski, J. Dopaminergic terminals in the rat cortex. Science 1973, 182, 499–501.
- Coyle, J.T.; Axelrod, J. Development of the uptake and storage of L- norepinephrine in the rat brain. J. Neurochem. 1971, 18, 2061–2075.
- Carlsson, A.; Dahlstroem, A.; Fuxe, K.; Lindqvist, M. Histochemical and biochemical detection of monoamine release from brain neurons. Life Sci. 1965, 4, 809–816.
- Anden, N.E.; Carlsson, A.; Dahlstroem, A.; Fuxe, K.; Hillarp, N.A.; Larsson, K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964, 3, 523–530.
- Rech, R.H.; Borys, H.K.; Moore, K.E. Alterations in behavior and brain catecholamine levels in rats treated with alpha-methyltyrosine. J. Pharmacol. Exp. Ther. 1966, 153, 412–419.
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Trans. 2019, 126, 397–409.
- Kumer, S.C.; Vrana, K.E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996, 67, 443–462.
- Reed, X.; Bandrés-Ciga, S.; Blauwendraat, C.; Cookson, M.R. The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol. Dis. 2019, 124, 230–239.
- Nishioka, K.; Imai, Y.; Yoshino, H.; Li, Y.; Funayama, M.; Hattori, N. Clinical Manifestations and Molecular Backgrounds of Parkinson’s Disease Regarding Genes Identified from Familial and Population Studies. Front. Neurol. 2022, 13, 764917.
- Chotibut, T.; Davis, R.W.; Arnold, J.C.; Frenchek, Z.; Gurwara, S.; Bondada, V.; Geddes, J.W.; Salvatore, M.F. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson’s model. Mol. Neurobiol. 2014, 49, 1282–1292.
- Pickel, V.M.; Beckley, S.C.; Joh, T.H.; Reis, D.J. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981, 225, 373–385.
- Moore, C.; Xu, M.; Bohlen, J.K.; Meshul, C.K. Differential ultrastructural alterations in the Vglut2 glutamatergic input to the substantia nigra pars compacta/pars reticulata following nigrostriatal dopamine loss in a progressive mouse model of Parkinson’s disease. Eur. J. Neurosci. 2020, 53, 2061–2077.
- Fiorenzato, E.; Antonini, A.; Bisiachhi, P.; Weis, L.; Biundo, R. Asymmetric Dopamine Transporter Loss Affects Cognitive and Motor Progression in Parkinson’s Disease. Mov. Disord. 2021, 36, 2303–2313.
- Beauchamp, L.C.; Dore, V.; Villemagne, V.L.; Xu, S.; Finkelstein, D.; Barnham, K.J.; Rowe, C. Utilizing 18F-AV-133 VMAT2 PET Imaging to Monitor Progressive Nigrostriatal Degeneration in Parkinson Disease. Neurology 2023, 101, e2314–e2324.
- Salvatore, M.F. ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J. Neurochem. 2014, 129, 548–558.
- Bezard, E.; Dovero, S.; Prunier, C.; Ravenscroft, P.; Chalon, S.; Guilloteau, D.; Crossman, A.R.; Bioulac, B.; Brotchie, J.M.; Gross, C.E. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned Macaque model of Parkinson’s disease. J. Neurosci. 2001, 21, 6853–6861.
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431.
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A Role for alpha -Synuclein in the Regulation of Dopamine Biosynthesis. J. Neurosci. 2002, 22, 3090–3099.
- Kasanga, E.A.; Han, Y.; Shifflet, M.K.; Navarrete, W.; McManus, R.; Parry, C.; Barahona, A.; Nejtek, V.A.; Manfredsson, F.P.; Kordower, J.H.; et al. Nigral-specific increase in ser31 phosphorylation compensates for tyrosine hydroxylase protein and nigrostriatal neuron loss: Implications for delaying parkinsonian signs. Exp. Neurol. 2023, 368, 114509.
- Johnson, M.E.; Salvatore, M.F.; Maiolo, S.A.; Bobrovskaya, L. Tyrosine hydroxylase as a sentinel for central and peripheral tissue responses in Parkinson’s progression: Evidence from clinical studies and neurotoxin models. Prog. Neurobiol. 2018, 165–167, 1–25.
- Shehadeh, J.; Double, K.I.; Murphy, K.E.; Bobrovskaya, L.; Reyes, L.; Dunkely, P.R.; Halliday, G.M.; Dickson, P.W. Expression of tyrosine hydroxylase isoforms and phosphorylation at serine 40 in the human nigrostriatal system in Parkinson’s disease. Neurobiol. Dis. 2019, 130, 104524.
- Nakashima, A.; Mori, K.; Kaneko, Y.S.; Hayashi, N.; Nagatsu, T.; Ota, A. Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Biochem. Biophys. Res. Commun. 2011, 407, 343–347.
- Kolacheva, A.; Alekperova, L.; Pavlova, E.; Bannikova, A.; Ugrumov, M.V. Changes in tyrosine hydroxylase activity and dopamine synthesis in the nigrostriatal system of mice in an acute model of Parkinson’s disease as a manifestation of neurodegeneration and neuroplasticity. Brain Sci. 2022, 12, 779.
- Haycock, J.W.; Haycock, D.A. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals: Multiple-site phosphorylation in vivo and in synaptosomes. J. Biol. Chem. 1991, 266, 5650–5657.
- Morgenroth, V.H.; Hegstrand, L.R.; Roth, R.H.; Greengard, P. Evidence for involvement of protein kinase in the activation by adenosine 3′:5′-monophosphate of brain tyrosine 3-monooxygenase. J. Biol. Chem. 1975, 250, 1946–1948.
- Willard, A.M.; Islett, B.R.; Whalen, T.C.; Mastro, K.J.; Ki, C.S.; Mao, X.; Gittis, A.H. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive depletion in mice. eLife 2019, 8, e42746.
- Kliem, M.A.; Maidment, N.T.; Axkerson, L.C.; Chen, S.; Smith, Y.; Wichmann, T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J. Neurophysiol. 2007, 98, 489–1500.
- Dagra, A.; Miller, D.R.; Lin, M.; Gopinath, A.; Shaerzadeh, F.; Harris, S.; Sorrentino, Z.A.; Stoier, J.F.; Velasco, S.; Azar, J.; et al. α-Synuclein-induced dysregulation of neuronal activity contributes to murine dopamine neuron vulnerability. NPJ Park. Dis. 2021, 7, 76.
- Matschke, L.A.; Komadowski, M.A.; Stohr, A.; Lee, B.; Henrick, M.T.; Griesbach, M.; Rinne, S.; Geibl, F.F.; Chiu, W.H.; Koprich, J.B.; et al. Enhanced firing of locus coeruleus neurons and SK channel dysfunction are conserved in distinct models of prodromal Parkinson’s disease. Sci. Rep. 2022, 12, 3180.
- Ellens, D.J.; Leventhal, D.K. Electrophysiology of Basal Ganglia and Cortex in Models of Parkinson Disease. J. Park.’s Dis. 2013, 3, 241–254.
- Lin, M.; Mckie, P.M.; Shaerzadeh, F.; Gamble-George, J.; Miller, D.R.; Martyniuk, C.J.; Khoshbouei, H. In Parkinson’s patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition. Acta Neuropathol. Commun. 2021, 9, 107.
- Matuskey, D.; Tinaz, S.; Wilcox, K.C.; Naganawa, M.; Toyonaga, T.; Dias, M.; Henry, S.; Pittman, B.; Ropchan, J.; Nabulsi, N.; et al. Synaptic Changes in Parkinson Disease Assessed with in vivo Imaging. Ann. Neurol. 2020, 87, 329–338.
- Saari, L.; Kivinen, K.; Gardberg, M.; Joutsa, J.; Noponen, T.; Kaasinen, V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 2017, 88, 1461–1467.
- Creed, R.B.; Menallel, L.; Casey, B.; Dave, K.D.; Janssens, H.B.; Veinbergs, I.; van der Hart, M.; Rassoulpour, A.; Goldberg, M.S. Basal and Evoked Neurotransmitter Levels in Parkin, DJ-1, PINK1 and LRRK2 Knockout Rat Striatum. Neuroscience 2019, 409, 169–179.
- Chotibut, T.; Fields, V.; Salvatore, M.F. Norepinephrine transporter inhibition with desipramine exacerbates L-DOPA-induced dyskinesia: Role for synaptic dopamine regulation in denervated nigrostriatal terminals. Mol. Pharmacol. 2014, 86, 675–685.
- Sarre, S.; Vandeneede, D.; Ebinger, G.; Michotte, Y. Biotransformation of L-DOPA to dopamine in the substantia nigra of freely moving rats: Effect of dopamine receptor agonists and antagonists. J. Neurochem. 1990, 70, 1730–1739.
- Perez, X.A.; Parameswaran, N.; Huang, L.Z.; O’Leary, K.T.; Wuik, M. Pre-synaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J. Neurochem. 2008, 105, 1861–1872.
- Mela, F.; Marti, M.; Bido, S.; Cenci, M.A.; Morari, M. In vivo evidence for a differential contribution of striatal and nigral D1 and D2 receptors to l-DOPA induced dyskinesia and the accompanying surge of nigral amino acid levels. Neurobiol. Dis. 2012, 45, 573–582.
- Kliem, M.A.; Pare, J.F.; Khan, Z.U.; Wichmann, T.; Smith, Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur. J. Neurosci. 2010, 31, 836–851.
- Mailman, R.B.; Yang, Y.; Huang, X. D1, not D2, dopamine receptor activation dramatically improves MPTP-induced parkinsonism unresponsive to levodopa. Eur. J. Pharmacol. 2021, 892, 173760.
- Trevitt, J.T.; Carlson, B.B.; Nowend, K.; Salamone, J.D. Substantia nigra pars reticulate is a highly potent site of action for the behavioral effects of the D1 antagonist SCH23390 in rat. Psychopharmacology 2001, 156, 32–41.
- Tang, P.; Knight, W.C.; Li, H.; Guo, Y.; Perlmutter, J.S.; Benzinger, T.L.S.; Morris, J.C.; Xu, J. Dopamine D1 + D3 receptor density may correlate with parkinson disease clinical features. Ann. Clin. Transl. Neurol. 2021, 8, 224–237.
- Roedter, A.; Winkler, C.; Samil, M.; Walter, G.; Brandis, A.; Nikkhah, G. Comparison of unilateral and bilateral intrastriatal 6-hydroxydopamine-induced axon terminal lesions: Evidence for interhemispheric functional coupling of the two nigrostriatal pathways. J. Comp. Neurol. 2001, 432, 217–229.
- Radnikow, G.; Misgeld, U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J. Neurosci. 1998, 18, 2009–2016.
- Dorval, A.D.; Grill, W.M. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 2014, 111, 1949–1959.
- DeLong, M.R.; Wichmann, T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol. 2015, 72, 1354–1360.
- McGregor, M.M.; Nelson, A.B. Circuit mechanisms of Parkinson’s disease. Neuron 2019, 101, 1042–1056.
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Di Filippo, M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat. Neurosci. 2014, 17, 1022–1030.
- Blesa, J.; Foffani, G.; Dehay, B.; Bezard, E.; Obeso, J.A. Motor and non-motor circuit disturbances in early Parkinson disease: Which happens first? Nat. Rev. Neurosci. 2022, 23, 115–128.
- Zaman, V.; Boger, H.A.; Granholm, A.C.; Rohrer, B.; Moore, A.; Buhusi, M.; Gerhardt, G.A.; Hoffer, B.J.; Middaugh, L.D. The nigrostriatal dopamine system of aging GFRalpha-1 heterozygous mice: Neurochemistry, morphology and behavior. Eur. J. Neurosci. 2008, 28, 1557–1568.
- Pruett, B.S.; Salvatore, M.F. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol. Neurobiol. 2013, 47, 988–999.
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595.
- Grondin, R.; Cass, W.A.; Zhang, Z.; Stanford, J.A.; Gash, D.M.; Gerhardt, G.A. Glial Cell Line-Derived Neurotrophic Factor Increases Stimulus-Evoked Dopamine Release and Motor Speed in Aged Rhesus Monkeys. J. Neurosci. 2003, 23, 1974–1980.
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255.
- Gerhardt, G.A.; Cass, W.A.; Huettl, P.; Brock, S.; Zhang, Z.; Gash, D.M. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999, 817, 163–171.
- Hoffer, B.J.; Hoffman, A.F.; Bowenkamp, K.E.; Huettl, P.; Hudson, J.; Martin, D.; Lin, L.F.; Gerhardt, G.A. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994, 182, 107–111.
- Lang, A.E.; Gill, S.S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson’s disease. Ann. Neurol. 2006, 59, 459–466.
- Salvatore, M.F.; Zhang, J.L.; Large, D.M.; Wilson, P.E.; Gash, C.R.; Thomas, T.C.; Haycock, J.W.; Bing, G.; Stanford, J.A.; Gash, D.M.; et al. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in rat striatum and substantia nigra. J. Neurochem. 2004, 90, 245–254.
- Salvatore, M.F.; Gerhardt, G.A.; Dayton, R.D.; Klein, R.L.; Stanford, J.A. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp. Neurol. 2009, 219, 197–207.
- Kasanga, E.A.; Han, Y.; Navarrete, W.; McManus, R.; Shifflet, M.K.; Parry, C.; Barahona, A.; Manfredsson, F.P.; Nejtek, V.A.; Richardson, J.R.; et al. Differential expression of RET and GDNF family receptor, GFR-α1, between striatum and substantia nigra following nigrostriatal lesion: A case for diminished GDNF-signaling. Exp. Neurol. 2023, 366, 114435.
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525.
- Kordower, J.H.; Goetz, C.G.; Chu, Y.; Halliday, G.M.; Nicholson, D.A.; Musial, T.F.; Marmion, D.J.; Stoessl, A.J.; Freeman, T.B.; Olanow, C.W. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann. Neurol. 2017, 81, 46–57.
- Furukawa, K.; Shima, A.; Kambe, D.; Nishida, A.; Wada, I.; Sakamaki, H.; Yoshimura, K.; Terada, Y.; Sakato, Y.; Mitsuhashi, M.; et al. Motor progression and nigrostriatal neurodegeneration in Parkinson’s disease. Ann. Neurol. 2022, 92, 110–121.
- Karimi, M.K.; Tian, L.; Flores, H.; Su, Y.; Tabbal, S.D.; Loftin, S.K.; Moerlin, S.M.; Perlmutter, J.S. Validation of nigrostriatal positron emission tomography measures: Critical limits. Ann. Neurol. 2013, 73, 390–396.
- Perlmuttter, J.S.; Norris, S.A. Neuroimaging biomarkers for Parkinson’s disease: Fact and fantasy. Ann. Neurol. 2014, 76, 769–783.
- Schröter, N.; Rijntjes, M.; Urbach, H.; Weiller, C.; Treppner, M.; Kellner, E.; Jost, W.H.; Sajonz, B.E.A.; Reisert, M.; Hosp, J.A.; et al. 2022. Disentangling nigral and putaminal contribution to motor impairment and levodopa response in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 132.
- Pérez-Taboada, I.; Alberquilla, S.; Martin, E.D.; Anand, R.; Vietti-Michelina, S.; Tebeka, N.N.; Cantley, J.; Cragg, S.J.; Moratalla, R.; Vallejo, M. Diabetes Causes Dysfunctional Dopamine Neurotransmission Favoring Nigrostriatal Degeneration in Mice. Mov. Disord. 2020, 35, 1636–1648.
- Gonzalez-Rodriguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656.
- Dave, K.D.; De Silva, S.; Sheth, N.P.; Ramboz, S.; Beck, M.J.; Quang, C.; Switzer, R.C., III; Ahmad, S.O.; Sunkin, S.M.; Walker, D.; et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol. Dis. 2014, 70, 190–203.
- Blesa, J.; Pifl, C.; Sánchez-González, M.A.; Juri, C.; García-Cabezas, M.A.; Adánez, R.; Iglesias, E.; Collantes, M.; Peñuelas, I.; Sánchez-Hernández, J.J.; et al. The nigrostriatal system in the presymptomatic and symptomatic stages in the MPTP monkey model: A PET, histological, and biochemical study. Neurobiol. Dis. 2012, 48, 79–91.
- Petzinger, G.M.; Walsh, J.P.; Akopian, G.; Hogg, E.; Abernathy, A.; Arevalo, P.; Turnquist, P.; Vuckovic, M.; Fisher, B.E.; Togasaki, D.M.; et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007, 27, 5291–5300.
- O’Dell, S.J.; Gross, N.B.; Fricks, A.N.; Casiano, B.D.; Nguyen, T.B.; Marshall, J.F. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience 2007, 144, 1141–1151.
- Churchill, M.J.; Pflibsen, L.; Sconce, M.D.; Moore, C.; Kim, K.; Meshul, C.K. Exercise in an animal model of Parkinson’s disease: Motor recovery but not restoration of the nigrostriatal pathway. Neuroscience 2017, 359, 224–247.
- Robertson, G.S.; Robertson, H.A. Evidence that L-DOPA-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 1999, 9, 3326–3331.
- Robertson, G.S.; Robertson, H.A. Evidence that the substantia nigra is a site of action for L-DOPA. Neurosci. Lett. 1988, 89, 204–208.
- Jackson, E.A.; Kelly, P.H. Role of nigral dopamine in amphetamine-induced locomotor activity. Brain Res. 1983, 278, 366–369.
- Bradbury, A.J.; Costall, B.; Kelly, M.E.; Naylor, R.J.; Smith, J.A. Biochemical correlates of motor changes caused by the manipulation of dopamine function in the substantia nigra of the mouse. Neuropharmacology 1985, 24, 1155–1161.
- Jackson, E.A.; Kelly, P.H. Effects of intranigral injections of dopamine agonists and antagonists, glycine, muscimol and N-methyl-D,L-aspartate on locomotor activity. Brain Res. Bull. 1984, 13, 309–317.
- Ahlenius, S.; Anden, N.E.; Engel, J. Restoration of locomotor activity in mice by low L-DOPA doses after suppression by alpha-methyltyrosine but not by reserpine. Brain Res. 1973, 62, 189–199.
- Dolphin, A.C.; Jenner, P.; Marsden, C.D. The relative importance of dopamine and noradrenaline receptor stimulation for the restoration of motor activity in reserpine or alpha-methyl-p-tyrosine pre-treated mice. Pharmacol. Biochem. Behav. 1976, 4, 661–670.
- Salvatore, M.F.; Pruett, B.S. Dichotomy of Tyrosine Hydroxylase and Dopamine Regulation between Somatodendritic and Terminal Field Areas of Nigrostriatal and Mesoaccumbens Pathways. PLoS ONE 2012, 7, e29867.
- Leng, A.; Mura, A.; Hengerer, B.; Feldon, J.; Ferger, B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav. Brain Res. 2005, 154, 375–383.
- Paquette, M.A.; Marsh, S.T.; Hutchings, J.E.; Castañeda, E. Amphetamine-evoked rotation requires newly synthesized dopamine at 14 days but not 1 day after intranigral 6-OHDA and is consistently dissociated from sensorimotor behavior. Behav. Brain Res. 2009, 200, 197–207.
- Ankenman, R.; Salvatore, M.F. Low dose alpha-methyl-para-tyrosine (AMPT) in the treatment of dystonia and dyskinesia. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 65–69.
- Bloemen, O.J.N.; de Koning, M.B.; Boot, E.; Booij, J.; van Amelsvoort, T.A. Challenge and Therapeutic Studies Using Alpha-Methyl-para-Tyrosine (AMPT) in Neuropsychiatric Disorders: A Review. Cent. Nerv. Syst. Agents Med. Chem. 2008, 8, 249–256.
- Rubinstein, M.; Gershanik, O.; Stefano, F.J. Different roles of D-1 and D-2 dopamine receptors involved in locomotor activity of supersensitive mice. Eur. J. Pharmacol. 1988, 148, 419–426.
- Lima, A.C.; Meurer, Y.S.R.; Bioni, V.S.; Cunha, D.M.G.; Goncalves, N.; Lopes-Silva, L.B.; Becegato, M.; Soares, M.B.L.; Marinho, G.F.; Santos, J.R.; et al. Female Rats Are Resistant to Cognitive, Motor and Dopaminergic Deficits in the Reserpine-Induced Progressive Model of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 757714.
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391.
- May, R.H.; Voegele, G.E. Parkinsonian reactions following chlorpromazine and reserpine; similar reactions in the same patients. AMA Arch. Neurol. Psychiatry 1956, 75, 522–524.
- Nirenberg, M.J.; Chan, J.; Liu, Y.; Edwards, R.H.; Pickel, V.M. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: Potential sites for somatodendritic storage and release of dopamine. J. Neurosci. 1996, 16, 4135–4145.
- Keller, C.M.; Salvatore, M.F.; Pruett, B.S.; Guerin, G.F.; Goeders, N.E. Biphasic dopamine regulation in mesoaccumbens pathway in response to non-contingent binge and escalating methamphetamine regimens in the Wistar rat. Psychopharmacology 2011, 215, 513–526.
- Nissbrandt, H.; Sundström, E.; Jonsson, G.; Hjorth, S.; Carolsson, A. Synthesis and Release of Dopamine in Rat Brain: Comparison Between Substantia Nigra Pars Compacta, Pars Reticulata, and Striatum. J. Neurochem. 1989, 52, 1170–1182.
- Heeringa, M.J.; Abercrombie, E.D. Biochemistry of Somatodendritic Dopamine Release in Substantia Nigra: An In Vivo Comparison with Striatal Dopamine Release. J. Neurochem. 1995, 65, 192–200.
- Santiago, M.; Westerink, B.H.C. Characterization and Pharmacological Responsiveness of Dopamine Release Recorded by Microdialysis in the Substantia Nigra of Conscious Rats. J. Neurochem. 1991, 57, 738–747.
- Yee, A.G.; Forbes, B.; Cheung, P.Y.; Martini, A.; Burrell, M.H.; Freestone, P.S.; Lipski, J. Action potential and calcium dependence of tonic somatodendritic dopamine release in the Substantia Nigra pars compacta. J. Neurochem. 2018, 148, 462–479.
- Cragg, S.J.; Rice, M.E. Dancing past the DAT at a DA synapse. Trends Neurosci. 2004, 27, 270–277.
- Kaasinen, V.; Vahlberg, T.; Stoessl, J.A.; Strafella, A.P.; Antonini, A. Dopamine receptors in Parkinson’s disease: A meta-analysis of imaging studies. Mov. Disord. 2021, 36, 1781–1791.
- Biswas, B.; Carlsson, A. Potentiation by Neuroleptic Agents of the Inhibitory Action of Intraperitoneally Administered GABA on the Locomotor Activity of Mice. Pharmacol. Biochem. Behav. 1978, 6, 651–654.
- Hillegaart, V.; Ahlenius, S. Effects of raclopride on exploratory locomotor activity, treadmill locomotion, conditioned avoidance behaviour and catalepsy in rats: Behavioural profile comparisons between raclopride, haloperidol and preclamol. Pharmacol. Toxicol. 1987, 60, 350–354.
- Löschmann, P.A.; Smith, L.A.; Lange, K.W.; Jaehnig, P.; Jenner, P.; Marsden, C.D. Motor activity following the administration of selective D-1 and D-2 dopaminergic drugs to normal common marmosets. Psychopharmacology 1991, 105, 303–309.
- Ericson, H.; Radesäter, A.C.; Servin, E.; Magnusson, O.; Mohringe, B. Effects of intermittent and continuous subchronic administration of raclopride on motor activity, dopamine turnover and receptor occupancy in the rat. Pharmacol. Toxicol. 1996, 79, 277–286.
- Hoffman, D.C.; Beninger, R.J. The D1 dopamine receptor antagonist, SCH 23390 reduces locomotor activity and rearing in rat. Pharmacol. Biochem. Behav. 1985, 22, 341–342.
- Schindler, C.W.; Caramona, G.N. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol. Biochem. Behav. 2002, 72, 857–863.
- Svensson, K.A.; Heinz, B.A.; Schaus, J.M.; Beck, J.P.; Hao, J.; Krushinski, J.H.; Reinhard, M.R.; Cohen, M.P.; Hellman, S.L.; Getman, B.G.; et al. An Allosteric Potentiator of the Dopamine D1 Receptor Increases Locomotor Activity in Human D1 Knock-In Mice without Causing Stereotypy or Tachyphylaxis. J. Pharmacol. Exp. Ther. 2017, 360, 117–128.
- Isaacson, S.H.; Hauser, R.A.; Pahwa, R.; Gray, D.; Duvvuri, S. Dopamine agonists in Parkinson’s disease: Impact of D1-like or D2-like dopamine receptor subtype selectivity and avenues for future treatment. Clin. Park. Relat. Disord. 2023, 9, 100212.
- Papapetropoulos, S.; Liu, W.; Duvvuri, S.; Thayer, K.; Gray, D.L. Evaluation of D1/D5 partial agonist PF-06412562 in Parkinson’s disease following oral administration. Neurodegener. Dis. 2018, 18, 262–269.
- Huang, X.; Lewis, M.M.; Van Scoy, L.J.; De Jesus, S.; Eslinger, P.J.; Arnold, A.C.; Miller, A.J.; Fernandez-Mendoza, J.; Snyder, B.; Harrington, W.; et al. The D1/D5 Dopamine Partial Agonist PF-06412562 in Advanced-Stage Parkinson’s Disease: A Feasibility Study. J. Park.’s Dis. 2020, 10, 1515–1527.
- Pothos, E.N.; Przedborski, S.; Davila, V.; Schmitz, Y.; Sulzer, D. D2-Like Dopamine Autoreceptor Activation Reduces Quantal Size in PC12 Cells. J. Neurosci. 1998, 18, 5575–5585.
- Cragg, S.J.; Greenfield, S.A. Differential Autoreceptor Control of Somatodendritic and Axon Terminal Dopamine Release in Substantia Nigra, Ventral Tegmental Area, and Striatum. J. Neurosci. 1997, 17, 5738–5746.
- Lanza, K.; Bishop, C. Dopamine D3 Receptor Plasticity in Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Biomedicines 2021, 9, 314.
- Chagraoui, A.; Di Giovanni, G.; De Deurwaerdère, P. Neurobiological and Pharmacological Perspectives of D3 Receptors in Parkinson’s Disease. Biomolecules 2022, 12, 243.
- Castello, J.; Cortes, M.; Malave, L.; Kottmann, A.; Sibley, D.R.; Friedman, E.; Rebholz, H. The Dopamine D5 receptor contributes to activation of cholinergic interneurons during L-DOPA induced dyskinesia. Sci. Rep. 2020, 10, 2542.
- Chetrit, J.; Taupignon, A.; Froux, L.; Morin, S.; Bouali-Benazzouz, R.; Naudet, F.; Kadiri, N.; Gross, C.E.; Bioulac, B.; Benazzouz, A. Inhibiting subthalamic D5 receptor constitutive activity alleviates abnormal electrical activity and reverses motor impairment in a rat model of Parkinson’s disease. J. Neurosci. 2013, 33, 14840–14849.
- Schultz, W.; Ruffieux, A.; Aebischer, P. The activity of pars compacta neurons of the monkey substantia nigra in relation to motor activation. Exp. Brain Res. 1983, 51, 377–387.
- da Silva, J.A.; Tecuapetla, F.; Paixão, V.; Costa, R.M. Dopamine neuron activity before action Initiation gates and invigorates future movements. Nature 2018, 554, 244–248.
- Coddington, L.T.; Dudman, J.T. Learning from Action: Reconsidering Movement Sinaling in Midbrain Dopamine Neuron Activity. Neuron 2019, 104, 63–77.
- Klaus, A.; da Silva, J.A.; Costa, R.M. What, If, and When to Move: Basal Ganglia Circuits and Self-Paced Action Initiation. Ann. Rev. Neurosci. 2019, 42, 459–483.
- Bergquist, F.; Shahabi, H.N.; Nissbrandt, H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003, 973, 81–91.
- Hebert, M.A.; Gerhardt, G.A. Normal and drug-induced locomotor behavior in aging: Comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998, 797, 42–54.
- Yurek, D.M.; Hipkens, S.B.; Hebert, M.A.; Gash, D.M.; Gerhardt, G.A. Age-related decline in striatal dopamine release and motoric function in Brown Norway/Fischer 344 hybrid rats. Brain Res. 1998, 791, 246–256.
- Gaugler, M.N.; Genc, O.; Bobela, W.; Mohanna, S.; Ardah, M.T.; El-Agnaf, O.M.; Cantoni, M.; Bensadoun, J.C.; Schneggenburger, R.; Knott, G.W.; et al. Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012, 123, 653–669.
- Goodwin, J.S.; Larson, G.A.; Swant, J.; Sen, N.; Javitch, J.A.; Zahniser, N.R.; De Felice, L.J.; Khoshbouei, H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 2009, 284, 2978–2989.
- Kahlig, K.M.; Binda, F.; Khoshbouei, H.; Blakely, R.D.; McMahon, D.G.; Javitch, J.A.; Galli, A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. USA 2005, 102, 3495–3500.
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433.
- Rivière, G.J.; Byrnes, K.A.; Gentry, W.B.; Owens, S.M. Spontaneous Locomotor Activity and Pharmacokinetics of Intravenous Methamphetamine and Its Metabolite Amphetamine in the Rat. J. Pharmacol. Exp. Ther. 1999, 291, 1220–1226.
- Laruelle, M.; Abi-Dargham, A.; van Dyck, C.H.; Rosenblatt, W.; Zea-Ponce, Y.; Zoghbi, S.S.; Baldwin, R.M.; Charney, D.S.; Hoffer, P.B.; Kung, H.F.; et al. SPECT Imaging of Striatal Dopamine Release after Amphetamine Challenge. J. Nuclear Med. 1995, 36, 1182–1190.
- Hall, D.A.; Stanis, J.J.; Avila, H.M.; Gulley, J.M. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: Evidence for qualitative differences in behavior. Psychopharmacology 2008, 195, 469–478.
- Ciliax, B.J.; Heilman, C.; Demchyshyn, L.L.; Pristupa, Z.B.; Ince, E.; Hersch, S.M.; Niznik, H.B.; Levey, A.I. The dopamine transporter: Immunochemical characterization and localization in brain. J. Neurosci. 1995, 15, 1714–1723.
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The Dopamine Transporter Is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci. 1996, 16, 436–437.
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, P489–P496.
- Hoffman, A.F.; Lupica, C.R.; Gerhardt, G.A. Dopamine transporter activity in the substantia nigra and striatum assessed by high-speed chronoamperometric recordings in brain slices. J. Pharmacol. Exp. Ther. 1998, 287, 487–496.
- Ford, C.P.; Gantz, S.C.; Phillips, P.E.M.; Williams, J.T. Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci. 2010, 30, 6975–6983.
- Cragg, S.J.; Rice, M.E.; Greenfield, S.A. Heterogeneity of Electrically Evoked Dopamine Release and Reuptake in Substantia Nigra, Ventral Tegmental Area, and Striatum. J. Neurophysiol. 1997, 77, 863–873.
- Ma, S.Y.; Ciliax, B.J.; Stebbins, G.; Jaffar, S.; Joyvce, J.N.; Cochran, E.J.; Kordower, J.H.; Mash, D.C.; Levey, A.I.; Mufson, E.J. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J. Comp. Neurol. 1999, 409, 25–37.
- Salvatore, M.F.; Apparsundaram, S.; Gerhardt, G.A. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol. Aging 2003, 24, 1147–1154.
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. NPJ Park. Dis. 2021, 7, 22.
- Jones, D.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034.
- Salvatore, M.F.; Calipari, E.S.; Jones, S.R. Regulation of tyrosine hydroxylase expression and phosphorylation in dopamine transporter-deficient mice. ACS Chem. Neurosci. 2016, 7, 941–951.
- Morón, J.A.; Brockiington, A.; Wise, R.A.; Rocha, B.A.; Hope, B.T. Dopamine Uptake through the Norepinephrine Transporter in Brain Regions with Low Levels of the Dopamine Transporter: Evidence from Knock-Out Mouse Lines. J. Neurosci. 2002, 22, 389–395.
- Chotibut, T.; Apple, D.M.; Jefferis, R.; Salvatore, M.F. Dopamine Transporter Loss in 6-OHDA Parkinson’s Model Is Unmet by Parallel Reduction in Dopamine Uptake. PLoS ONE 2012, 7, e52322.
- Reinwald, J.R.; Gass, N.; Mallien, A.S.; Sartorius, A.; Becker, R.; Sack, M.; Falfan-Melgoza, C.; von Hohenberg, C.C.; Leo, D.; Pfeiffer, N.; et al. Dopamine transporter silencing in the rat: Systems-level alterations in striato-cerebellar and prefrontal-midbrain circuits. Mol. Psychiatry 2022, 27, 2329–2339.
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612.
- Spielewoy, C.; Roubert, C.; Hamon, M.; Nosten-Bertrand, M.; Betancur, C.; Giros, B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 2000, 11, 279–290.
- Stanford, J.A.; Vorontsova, E.; Surgener, S.P.; Gerhardt, G.A.; Fowler, S.C. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: Exacerbation by nomifensine. Neurosci. Lett. 2002, 333, 195–198.
- Salvatore, M.F.; Kasanga, E.A.; Kelly, D.P.; Venable, K.E.; McInnis, T.R.; Cantu, M.A.; Terrebonne, J.; Lanza, K.; Meadows, S.M.; Centner, A.; et al. Modulation of nigral dopamine signaling mitigates parkinsonian signs of aging: Evidence from intervention with caloric restriction or inhibition of dopamine uptake. GeroScience 2023, 45, 45–63.
- Collier, T.J.; Lipton, J.; Daley, B.F.; Palfi, S.; Chu, Y.; Sortwell, C.; Bakay, R.A.; Sladek, J.R.; Kordower, J.H. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: Diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007, 26, 56–65.
- Pifl, C.; Hornykiewicz, O. Dopamine turnover is upregulated in the caudate/putamen of asymptomatic MPTP-treated rhesus monkeys. Neurochem. Int. 2006, 49, 519–524.
- Zigmond, M.J. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol. Dis. 1997, 4, 247–253.
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Lopez-Gonzalez del Rey, N.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161.
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301.
- Chu, Y.; Hirst, W.D.; Federoff, H.J.; Harms, A.S.; Stoessl, A.J.; Kordower, J.H. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain 2023, awad388.
- Sarre, S.; Yuan, H.; Jonkers, N.; Van Hemelrijck, A.; Ebinger, G.; Michotte, Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004, 90, 29–39.
