Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Eleonora Russo.
Natural deep eutectic solvents (NaDES) represent a new generation of green, non-flammable solvents, useful as an efficient alternative to the well-known ionic liquids. They can be easily prepared and exhibit unexpected solubilizing power for lipophilic molecules, although those of a hydrophilic nature are mostly used. For their unique properties, they can be recommend for different cosmetic and pharmaceutical applications, ranging from sustainable extraction, obtaining ready-to-use ingredients, to the development of biocompatible drug delivery responsive systems.
- natural deep eutectic solvents
- NaDES and formulations
- NaDES and drug delivery
- NaDES and cosmetic
- bioactive compound extraction
1. Introduction
Currently, interest in the development of sustainable processes and green bioactive compounds from renewable sources is steadily increasing in the cosmetic and pharmaceutical fields. From an extractive point of view, avoiding unfriendly solvents, saving sources and energy, and recycling waste have become primary objectives for the pharmaceutic and cosmetic context, according to the green extraction principles [1]. Conventional organic solvents are commonly used for extracting aromas, perfumes, medicines, and dyes from plants, but they are often not sustainable due to toxicity, high environmental impact and flammability [2]. For this reason, in recent years, research in the green extraction context has focused its attention on new non-toxic, biodegradable green solvents [3].
In this context, ionic liquids (ILs) and deep eutectic solvents (DES) can represent an excellent alternative to conventional hazardous organic solvents [2]. ILs are defined as salts deriving from the combination of an organic cation and an anion, characterized by a melting point below 100 °C, being in most cases liquids at room temperature [4]. DESs are defined as homogeneous eutectic mixtures obtained by mixing two or more pure components (liquids or solids, ions or neutral molecules) acting as hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD) [5]. High thermal stability, low volatility, and wide ranges of viscosity and polarity are some of the most interesting properties belonging to both ILs and DES [6,7][6][7]. In particular, the class of IL organic salts is characterized by low melting point and minimal vapor pressure, and they can be modified in terms of polarity and selectivity for different applications such as chemical or enzymatic reactions [8,9][8][9]. Unfortunately, their use is restricted due to their high toxicity and high production costs, including those for synthesis, purification and disposal [10]. These limitations can be overcome by deep eutectic solvents with comparable or better physical properties and phase behaviors than ILs [11]. First introduced by Abbott et al. [12], DES represent a great and successful alternative to ILs, characterized by easy preparation, purity and low costs [13]. The process to obtain DES involves the simple mixing of a hydrogen-bond acceptor (HBA) (like a quaternary ammonium salt) and a hydrogen-bond donor (HBD) at a suitable molar ratio [14]. Their interaction gives rise to supramolecular compounds, with peculiar chemical-physical characteristics [15[15][16],16], with a charge delocalization that is responsible for the lowering of the mixture melting temperature with respect to the individual components (generally from room temperature to 70 °C) [7]. Unfortunately, the use of DES, like green solvents at room temperature, can be hindered by their melting points being too high [17].
In this regard, a new generation of greener DES of natural origin has emerged over the past decade. In nature, it has been hypothesized that in plants, different metabolites can form eutectic mixtures which play different biological roles. They can act as an alternatives to water and lipids, with the ability to transport water-insoluble compounds inside the cells, explaining the co-presence of water soluble and insoluble compounds in the botanical matrix. For this reason, when these metabolites (i.e., sugars, alcohols, amino acids, organic acids) form DES, they are called “Natural DES” (NaDES) [18]. Their green properties and behaviors were first described by Choi et al. in 2011 [17,18,19][17][18][19].
Synthetic NaDES can reproduce this natural behavior, and they are considered promising new green solvents to be applied in several fields, such as in the cosmetic, pharmaceuticals and food areas. NADES were used successfully to extract phenolic compounds from plant material. For example, in recent years, research has been carried out on the NaDES extraction of phlorotannins from the brown alga Fucus vesiculosus L. [20,21][20][21]. Phlorotannins are polyphenols with antioxidant, anti-inflammatory, antiallergic, antibacterial, and antitumor properties. They have a wide range of cosmetic applications, e.g., in sunscreens as anti-aging and UV-protective agents, and in in food packaging films as preservatives. Some authors have suggested the use of NaDES as solvents to stabilize proteins (lysozyme, amylase, photosynthetic enzymes) and DNA [18,22,23][18][22][23]. This opportunity led to an increasing interest in NaDES as drug delivery systems for active, but poorly soluble, ingredients [24,25,26][24][25][26]. NaDES show a wide polarity range and high solubilization strength for a variety of compounds. They present several advantages over classical solvents, ILs, and DES, such as natural origin, low cost, biodegradability, absence of toxicity, sustainability, and simple preparation [22]. Although NaDES are recognized as being slightly toxic and with a low environmental impact, it must be mentioned that they show the phenomenon of eutrophication [27]. As extractive alternative solvents, NaDES allow the achievement of efficient extractions when compared to conventional solvents [28,29,30,31][28][29][30][31]. Moreover, they often improve the stability and storage of the extracted compounds of interest, such as phenols, β-carotene, and α-tocopherol [22,23,29,32,33][22][23][29][32][33].
2. NaDES Preparation
Many NaDES mixtures are biodegradable and have low toxicity [55,56[34][35][36],57], partially due to their natural origin. Most of their components present an intrinsic cosmetic or pharmaceutical activity, being well-known and used ingredients (organic acids, sugars, alcohols and polyols, amino acids and quaternary ammonium salts).
Particularly from a cosmetic point of view, this aspect presents many advantages: increasing the naturality of the compositions and the concentration of active ingredients, stabilizing them without adding preservatives, reducing the number of ingredients and allowing a synergistic effect to improve the biological activity of the formulation.
The preparation of NaDES yields easy results with high purity and no waste formation [58][37] according to the fundamental principles of green chemistry [59][38].
As mentioned above, NaDES can be prepared by mixing an HBA (i.e., choline chloride, choline acetate or betaine) with an HBD (glycerol, urea, glucose, sorbitol, fructose, etc.), with or without water [17], mainly applying these most common and different physical methods:
-
Heating and stirring method [17], where two components are mixed with a magnetic stirring bar, in a 50 °C water bath until a clear viscous liquid is formed, about 30–90 min later [17,22,60,61][17][22][39][40]. Otherwise, it is possible to follow the conditions stated by Abbot et al. 2003 [12], or heating at 80 °C under continuous stirring [60,62,63][39][41][42].
-
Evaporation method [17], which involves the use of rotary evaporator to allow the components’evaporation and dissolution in water at 50 °C. The liquid that is obtained is transferred to a silica gel desiccator until it reaches a constant weight.
The methods mentioned above are shown in Figure 21. The microwave-assisted preparation of NaDES represents a promising green technique, due to its advantages such as higher yields, lower energy consumption and shorter reaction times [68][47].
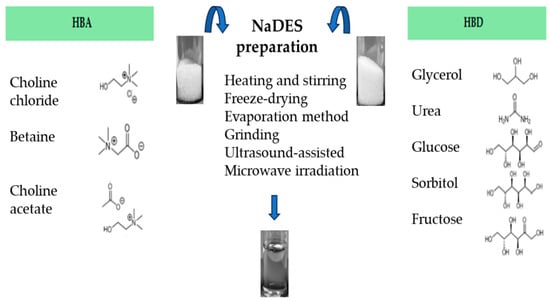
Figure 21.
Different preparation methods to obtain NaDES.
3. NaDES Structure
The structure and properties of NaDES are conferred by the type and ratio of components and also by the hydrogen (H) bonds established between the metabolites themselves [17,69,70][17][48][49]. The H bonds’strength is related to the phase-transition temperature, stability and solvent properties of the mixture [64][43]; their key roles in important NaDES features and behaviors (such as stability and formation) depend on their number and location [17].
The lowering of the mixture melting temperature, with respect to the single components, is due to the formation of a charge delocalization between the HBA and HBD and to the van der Waals forces that allow blocking the crystallization of the compounds [29]. Usually, a low freezing point can be determined by a higher binding capacity between the HBD and HBA [70][49].
The NaDES structures have been evaluated through nuclear magnetic resonance spectroscopy (NMR) studies, crystallographic data, fast atom bombardment-mass spectrometry (FAB)-MS and Fourier transform infrared spectroscopy (FT-IR) [17,60,71][17][39][50]. Thanks to the nuclear Overhauser effect spectroscopy (NOESY) spectra obtained, it has been seen that NaDES are characterized by a supramolecular structure mainly due to bonds established between HBAs and HBDs [17]. This supramolecular structure changes after water dilution [59][38]; in fact, it was observed that the presence or absence of water plays a significant role. This behavior occurs because the H bond systems that NaDES are able to form, will gradually fade when diluted with water, until disappearing when the water amount exceeds 50% v/v. In this regard, it has been observed that the degradation of concentrated NaDES was slower than that of diluted ones [72][51].
The amount of added water tolerated by the eutectic system should be determined for each NaDES. Moreover, the types of components used to form NaDES can influence their physicochemical properties, such as viscosity, conductivity, density, and polarity [60][39].
Craveiro et al. have demonstrated that water can increase polarity, which affects the solubility of NaDES [73][52]. Simultaneously, dilution with water can result in a decrease in viscosity. This rheological behavior is one of the main problems that NaDES present [17]. A high viscosity interferes with the flow of substances and decreases the extraction efficiency [74][53]; this problem can be overcome by heating. The high temperature and thermal expansion lead to increased molecular force and to structural damage, respectively [75][54]. Another way to reduce the viscosity is dilution with water, since, as already mentioned, water leads to the breaking of the hydrogen bonds and consequently to a lower viscosity [60][39].
Several works in the literature describe the use of NaDES to obtain, from natural sources, bioactive compounds that can be used in cosmetic and pharmaceutical formulations (Figure 32). The main advantage consists of the possibility of directly adding the NaDES-based extract itself to all types of topical formulations, both in the cosmetic and pharmaceutical fields, without dramatic changes in the rheological properties or sensorial profile [76][55]. However, only a few can actually be used for cosmetic applications because of safety or regulatory issues [75][54].
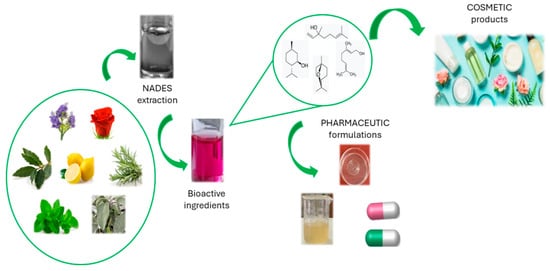
Figure 32.
Use of NaDES in cosmetic and pharmaceutical fields.
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627.
- Dai, Y. Natural Deep Eutectic Solvents and Their Application in Natural Product Research and Development. 2013. Available online: https://scholarlypublications.universiteitleiden.nl/handle/1887/21787 (accessed on 7 January 2024).
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Solvatochromic Parameters of Deep Eutectic Solvents Formed by Ammonium-Based Salts and Carboxylic Acids. Fluid. Phase Equilibria 2017, 448, 15–21.
- Osch, D.J.G.P.; van Kollau, L.J.B.M.; Bruinhorst, A.; van den Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biomass Fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665.
- Mero, A.; Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A.; Guazzelli, L. Comparison of Physicochemical and Thermal Properties of Choline Chloride and Betaine-Based Deep Eutectic Solvents: The Influence of Hydrogen Bond Acceptor and Hydrogen Bond Donor Nature and Their Molar Ratios. J. Mol. Liq. 2023, 377, 121563.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425.
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084.
- Visser, A.E.; Swatloski, R.P.; Rogers, R.D. pH-Dependent Partitioning in Room Temperature Ionic Liquids Provides a Link to Traditional Solvent Extraction Behavior. Green Chem. 2000, 2, 1–4.
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New Horizons in the Extraction of Bioactive Compounds Using Deep Eutectic Solvents: A Review. Anal. Chim. Acta 2017, 979, 1–23.
- El Abedin, S.Z.; Endres, F. Ionic Liquids: The Link to High-Temperature Molten Salts? Acc. Chem. Res. 2007, 40, 1106–1113.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 1, 70–71.
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected Issues Related to the Toxicity of Ionic Liquids and Deep Eutectic Solvents—A Review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992.
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Wagle, D.V.; Deakyne, C.A.; Baker, G.A. Quantum Chemical Insight into the Interactions and Thermodynamics Present in Choline Chloride Based Deep Eutectic Solvents. J. Phys. Chem. B 2016, 120, 6739–6746.
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68.
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705.
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Obluchinskaya, E.; Daurtseva, A.; Pozharitskaya, O.; Flisyuk, E.; Shikov, A. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus Vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263.
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents Providing Enhanced Stability of Natural Colorants from Safflower (Carthamus Tinctorius). Food Chem. 2014, 159, 116–121.
- Xin, R.; Qi, S.; Zeng, C.; Khan, F.I.; Yang, B.; Wang, Y. A Functional Natural Deep Eutectic Solvent Based on Trehalose: Structural and Physicochemical Properties. Food Chem. 2017, 217, 560–567.
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531.
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921.
- Mano, F.; Aroso, I.M.; Barreiros, S.; Borges, J.P.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Production of Poly(Vinyl Alcohol) (PVA) Fibers with Encapsulated Natural Deep Eutectic Solvent (NADES) Using Electrospinning. ACS Sustain. Chem. Eng. 2015, 3, 2504–2509.
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are Natural Deep Eutectic Solvents Always a Sustainable Option? A Bioassay-Based Study. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279.
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173.
- Fernández, M.d.L.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural Deep Eutectic Solvents-Mediated Extractions: The Way Forward for Sustainable Analytical Developments. Anal. Chim. Acta 2018, 1038, 1–10.
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-Functioning Deep Eutectic Solvents as Extraction and Storage Media for Bioactive Natural Products That Are Readily Applicable to Cosmetic Products. J. Clean. Prod. 2017, 151, 87–95.
- Liu, W.; Zhang, K.; Qin, Y.; Yu, J. A Simple and Green Ultrasonic-Assisted Liquid–Liquid Microextraction Technique Based on Deep Eutectic Solvents for the HPLC Analysis of Sesamol in Sesame Oils. Anal. Methods 2017, 9, 4184–4189.
- Milano, F.; Giotta, L.; Guascito, M.R.; Agostiano, A.; Sblendorio, S.; Valli, L.; Perna, F.M.; Cicco, L.; Trotta, M.; Capriati, V. Functional Enzymes in Nonaqueous Environment: The Case of Photosynthetic Reaction Centers in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 7768–7776.
- Zahrina, I.; Nasikin, M.; Krisanti, E.; Mulia, K. Deacidification of Palm Oil Using Betaine Monohydrate-Based Natural Deep Eutectic Solvents. Food Chem. 2018, 240, 490–495.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51.
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69.
- Pena-Pereira, F.; Kloskowski, A.; Namieśnik, J. Perspectives on the Replacement of Harmful Organic Solvents in Analytical Methodologies: A Framework toward the Implementation of a Generation of Eco-Friendly Alternatives. Green Chem. 2015, 17, 3687–3705.
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312.
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19.
- Martins, M.; Aroso, I.M.; Reis, R.L.; Duarte, A.R.C.; Craveiro, R.; Paiva, A. Enhanced Performance of Supercritical Fluid Foaming of Natural-Based Polymers by Deep Eutectic Solvents. AIChE J. 2014, 60, 3701–3706.
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of Natural Deep Eutectic Solvents for Extraction and Determination of Phenolics in Cajanus Cajan Leaves by Ultra Performance Liquid Chromatography. Sep. Purif. Technol. 2015, 149, 237–244.
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Fast and Green Extraction and Separation of Main Bioactive Flavonoids from Radix Scutellariae. Ind. Crops Prod. 2015, 63, 175–181.
- Gutiérrez, M.; Ferrer, M.L.; Yuste, L.; Rojo, F.; Monte, F. Bacteria Incorporation in Deep-Eutectic Solvents through Freeze-Drying. Angew. Chem. 2010, 49, 2158–2162.
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.C.; Marrucho, I.M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid–Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895.
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344.
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A Greener Approach to Prepare Natural Deep Eutectic Solvents. ChemistrySelect 2018, 3, 6122–6125.
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds; Food Engineering Series; Editions Springer: Berlin/Heidelberg, Germany, 2013.
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora Japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755.
- Gutiérrez, M.; Ferrer, M.L.; Mateo, C.R.; Monte, F. Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir ACS J. Surf. Colloids 2009, 25, 5509–5515.
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085.
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592.
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540.
- Mjalli, F.S.; Al-Azzawi, M. Aliphatic Amino Acids as Possible Hydrogen Bond Donors for Preparing Eutectic Solvents. J. Mol. Liq. 2021, 330, 115637.
- Benoit, C.; Virginie, C.; Boris, V. Chapter Twelve—The Use of NADES to Support Innovation in the Cosmetic Industry. In Advances in Botanical Research; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Eutectic Solvents and Stress in Plants; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 309–332.
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojcic Redovnikovic, I.; Duarte, A. Review of Deep Eutectic Systems from Laboratory to Industry, Taking the Application in the Cosmetics Industry as an Example. J. Clean. Prod. 2022, 380, 135147.
More
