Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Grzegorz P Łysiak.
The cornelian cherry is a plant that annually provides fruits, drupe-type, ranging in color from yellow through pink, red, carmine, and almost black. Cornelian cherry bears abundant fruit in temperate climate conditions, which means that its dark-colored fruits can be treated as an excellent source of anthocyanins. After consuming, anthocyanins have a protective function in the human body. Raw fruit extracts and their pure isolates, rich in anthocyanins, have a wide spectrum of health-promoting properties.
- healthy properties of fruit
- antidiabetic
- anticancer
- antioxidant
- antimicrobial properties
1. Bioavailability and Bioefficacy of Anthocyanins Derived from Cornelian Cherry
Anthocyanins are responsible for fruit color from pink through red to nearly black. Therefore, their level is the highest in dark-colored fruit: from 200 to 1560 mg·100 g−1 FW in elderberry, from 506 to 1000 mg·100 g−1 FW in chokeberry, from 8 to 750 mg·100 g−1 FW in red grapes, from 82.5 to 530 mg·100 g−1 FW in blueberry, and from130 to 400 mg·100 g−1 FW in blackcurrant [21][1]. Cornelian cherries contain from 5.8 to 442.11 mg·100 g−1 FW of anthocyanins [22,23][2][3]. Different anthocyanin content of individual fruit cultivars is also associated with their different color [24][4]. Yellow-colored cornelian cherries do not contain anthocyanins at all [25][5]. Over 650 different anthocyanins have been isolated in plants with six possible aglycon structures (anthocyanidins) most frequently occurring in natural conditions: pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin [26][6]. Qualitative analyses of anthocyanins in cornelian cherry most often reveal the presence of cyanidin 3-O-galactoside [27[7][8][9][10],28,29,30], cyanidin 3-O-robinobioside [25,31,32][5][11][12], pelargonidin 3-O-galactoside [27[7][8],28], pelargonidin 3-O-robinobioside [25,31][5][11] and delphinidin 3-O-galactoside [29,33][9][13]. In addition, there are peonidin 3-O-glucoside [33,34][13][14] and petunidin 3-O-glucoside [31[11][13],33], with no reports of anthocyanins derived from malvidin (Figure 1).
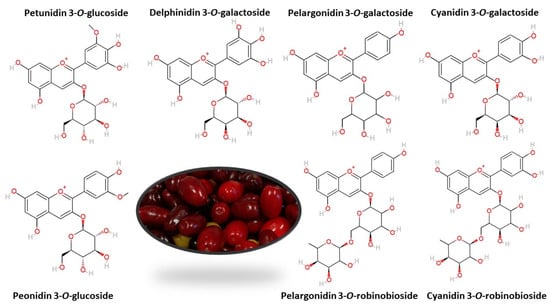
Figure 1.
The most common anthocyanins in cornelian cherry fruits.
Anthocyanins are among the few plant polyphenols that can be determined in plasma in their original, intact form as glycosides. Until recently, it was believed that anthocyanins have very poor bioavailability, and <1% of their consumed amount reaches the plasma. However, the estimation of bioavailability is limited by the lack of identification of anthocyanin metabolites and degradation products [10,35][15][16].
The biological activity of anthocyanins depends on their absorption by the human body and on metabolic processes. Anthocyanins show hydrophobic and electrostatic interactions with human albumin, preferred by hydroxyl groups in contrast to methyl groups. Studies demonstrate limited absorption of these compounds from food, as their concentration in blood plasma ranges from nM to μM. Researchers argue that anthocyanin glycosides are absorbed by a peculiar carrier—most likely a Na+ ion-dependent glucose transporter [36][17]. Anthocyanins and their metabolites remain in urine for up to 24 h after intake. Another factor involved in anthocyanin absorption is bilitranslocase—a plasma membrane carrier of organic anions found in the epithelial cells of the gastric mucosa [37][18]. Alternatively, they may also transform into glucuronide or sulfo-conjugate derivatives. Anthocyanin absorption takes place mainly in the stomach and small intestine [38][19]. Enzymes responsible for anthocyanin biotransformation include UDP-glucuronyl transferase, UDP–glucose dehydrogenase, and catechol-O-methyltransferase (COMT). They are present in the liver, small intestine, and kidneys and—depending on the chemical structure of anthocyanins—can modify them in various ways [39][20]. Due to this, both—primary anthocyanins and their secondary metabolites—can be detected in human urine and blood [40][21]. Differences in the concentrations of anthocyanins and their metabolites in the urine suggest that absorption of these pigments depends on their chemical structure, type and levels of substituted sugar radicals, and acylation method. Researchers demonstrated that binding with human albumin is more favorable for anthocyanins than it is for anthocyanidins [2][22]. It should be underlined that anthocyanin uptake and utilization also depend on external factors and individual traits such as age and different levels of stress [41][23].
David et al. [42][24] discovered that anthocyanins in cornelian cherries can remain in the human body for a long time. Their study, simulating a digestive process in vitro, evaluated the stability of anthocyanins derived from cornelian cherry during passage through the upper alimentary tract. Cornelian cherry extract was rich in anthocyanins such as cyanidin 3-O-galactoside, pelargonidin 3-O-glucoside, and pelargonidin 3-O-rutinoside. They demonstrated that gastric digestion had no significant effect on the levels of anthocyanins, and only intestinal digestion materially reduced their content and antioxidant activity. These discoveries suggest that cornelian cherries are an important source of anthocyanins in a human diet. They can have a beneficial effect on gastric health, while the products of their degradation and their metabolites can act as antioxidants in the small intestine. High levels of ingredients in fruits do not always go hand in hand with the human body’s capability of utilizing them.
2. Health-Promotion Effects of Anthocyanins Derived from Cornelian Cherry
The beneficial effects of anthocyanins from cornelian cherry fruit have been studied in in vitro (Table 1), in vivo (Table 2), and human experiments (Table 3).
Table 1.
Health-promoting properties of cornelian cherries anthocyanins proven in in vitro tests presented according to the PICO scheme.
| Health-Promoting Properties | Literature | Cells Used in the Study (Population) |
|---|
Table 2.
Health-promoting properties of cornelian cherries and anthocyanins proven in vivo presented according to the PICO scheme.
| Effects | Literature | Animals Subjected to Tests (Population) | Study Treatment (Intervention) | Study Treatment | Effects | Literature |
Type of Patients (Population)Control Treatment (Comparison) | (Intervention) | Control TreatmentMain Findings (Outcome) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Comparator) | Main Findings (Outcome) | |||||||||||||||||
| Dose of Cornelian Cherry Extract and Period of Its Intake (Intervention) | Control Treatment (Comparator) | Main Findings | (Outcome) | |||||||||||||||
| Anti-inflammatory activity | [43][25] | Ram seminal vesicles | Anthocyanins of C. mas—juice: delphinidin 3-O-‚-galactopyranoside 280 ppm, cyanidin 3-O-‚galactopyranoside 1079 ppm, and pelargonidin 3-O-‚-galactopyranoside 710 ppm | Ibuprofen, naproxen, | Ibuprofen and naproxen showed 47.5 and 54.3% of COX-I and 39.8 and 41.3% of COX-II inhibitory activities, respectively, at 10 μM concentrations. Anthocyanins 1, 2, and 3 displayed 9.2, 7.6, and 5.3% COX-I and 11.7, 12.4, and 7.8% COX-II activities, respectively. | |||||||||||||
| Antioxidant activity | [48][30] | 8–12 weeks male NMRI mice treated with Methotrexate (MTX) | CMFE (250, 500, 1000 mg·kg−1) and Vitamin E (100 IU kg−1) | |||||||||||||||
| Protective effect on the liver | [68][50] | physiologic saline | Patients with non-alcoholic fatty liver disease (NAFLD) | Both Vit E and CMFE were able to protect from MTX-induced effects on sperm maturity and DNA damage. | CMFE (320 mg·d−1 anthocyanins; 12 weeks). | Control group received the placebo, matched with the extract in terms of appearance, taste, color, and texture (but without any anthocyanins) for 12 weeks | Results indicated that anthocyanins had some impacts on NAFLD. | |||||||||||
| Anticancer activity | [44][26] | Lung non-small cell cancer; breast adenocarcinoma cell; ovarian cancer cell; prostate adenocarcinoma cell | Hydro-alcoholic extract of | |||||||||||||||
| Protective effect on the heart | C. mas: | |||||||||||||||||
| 0, 5, 20, 100, 250, 500, 1000 μg/mL | Negative control (cells in RPMI-1640 medium) | The mean growth inhibition was 81.8%, 81.9%, 81.6%, and 79.3% in ovarian cancer, breast adenocarcinoma, prostate adenocarcinoma, and lung non-small cell cancer, respectively. | ||||||||||||||||
| [ | 49][31] | [69][51Rats treated with carbon tetrachloride (CCl4) | ]Pre and post-treatment CMFE 300 and 700 mg·kg−1 | Control group without CMFE | CMFE significantly decreased the increased levels of serum lactate dehydrogenase, serum creatine kinase, and myocardial lipid peroxides and significantly increased the myocardial endogenous antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) levels. | Patients with non-alcoholic fatty liver disease (NAFLD) | CMFE (320 mg·d−1 anthocyanins; 12 weeks). | Control group received the placebo, matched with the extract in terms of appearance, taste, color, and texture (but without any anthocyanins) for 12 weeks | No significant impact of CMFE on serum ALT and AST levels, as well as hepatic steatosis among NAFLD patients. A significant reduction was observed in the levels of CK-18 among the CMFE group at the end of the study. No significant difference was found between the CMFE and placebo groups with regard to this marker. Fibrosis score increased significantly in the placebo group at the end of the study. | [45][27] | Breast adenocarcinoma, cervix epithelioid carcinoma, lung adenocarcinoma | containing different amounts of anthocyanins depending on growth locations CC1 0.89, CC2 0.80, CC3 1.40, CC4 1.08 mg CyGE·g−1 FW | ||||||
| Protective effect on the liver | [50][ | |||||||||||||||||
| Antihyperglycemic | 32 | Negative control (Cells in DMEM medium) (PAA Laboratories GmbH, Pashing, Austria) | ] | The antiproliferative activity of cornelian cherry fruit extracts depended on growth locations. Wild cornelian cherry (CC3) from Drinić had the highest monomeric anthocyanin content and the highest inhibition of free radicals (IC50DPPH = 262.19 mg/mL; IC50ABTS = 76.78 mg/mL; IC50OH˙ = 102.31 mg/mL) and inhibition of breast adenocarcinoma cell line growth (IC50MCF-7 = 1.37 mg/mL). | ||||||||||||||
| [ | Wistar strain male albino rats treated with carbon tetrachloride | 70][52] | CMFE 200 and 500 mg·kg−1 and Silymarin 100 mg·kg−1 | irrigated with drinking water | Oral administration of CMFE provided significant liver protection by reducing elevated serum enzyme levels, total serum protein, albumin, and hepatic lipid peroxidation content. | Patients with type 2 diabetes mellitus | 300 mg d−1 | Antihyperglycemic effects | [ | |||||||||
| anthocyanins; 6 weeks | Placebo capsules; 6 weeks | Significant increase in insulin levels and a decrease in HgbA1 C and TG levels was observed in the drug group compared to the placebo. | [51][33]46 | |||||||||||||||
| Antihyperlipidemic effects | ][28] | Rodent pancreatic β-cells (INS-1 832/13) | [Purified delphinidin-3-glucoside from | Wistar strain male albino rats treated with carbon tetrachloride71C. officinalis | ][53]CMFE 200 and 500 mg·kg−1 per 16 days and CMFE 200 and 500 mg·kg−1 administered 2, 6, 12, 24 and 48 h after CCl4 toxication fruits. cyanidin-3-galactoside and pelargonidin-3-galactoside from C. mas fruits: 5, 10, 50, 100 and 250 μg·mL−1. | Negative control (cells in RPMI-1640 medium) | irrigated with drinking water | The activities of antioxidant enzymes (MDA, CAT, SOD, GPx) in the CCl4-treated group were lower than those in the normal control. The activity of these enzymes in the CMFE-treated groups increased significantly compared to the toxic group. | Dyslipidemic children and adolescentsDelphinidin 3-O-glucoside, cyanidin 3- | 50 g of CMFE twice a day after lunch and dinner, 6 weeksO-galactoside, and pelargonidin 3- | Diet without CMFE, 6 weeksO-galactoside were distinguished as the most effective anthocyanins to stimulate insulin secretion. | After week 6 of the trial, the TC, TG, LDL-C, apo B, ICAM-1, and VCAM-1 levels in the CMFE group were significantly lower, and the HDL-C and apo A-I levels were higher than at baseline. | Antihyperlipidemic effects | [47][29] | Porcine pancreas powder | Water and ethanolic extract of C. mas and C. alba; pelargonidin 3-O-galactoside: 7.5 μg·mL−1 | Negative control (Porcine pancreas powder in Tris-HCl buffer, pH 8.0) | The most active anthocyanin in the inhibition of pancreatic lipase activity was pelargonidin 3-O-galactoside. |
CC1—cornelian cherry from Ljubomir, CC2—cornelian cherry from Koravlica, CC3—cornelian cherry from Drinić, CC4 cornelian cherry from Drvar, FW—fresh weight.
| [ | |||||||||
| 52 | |||||||||
| ] | |||||||||
| [34] | Wistar strain male albino rats treated with methotrexate (MTX) | CMFE 700 mg·kg−1 per 7 days and Mice first day treated with MTX and then treated with 300, 700, and 1400 mg for 7 days | physiologic saline | Rats treated with MTX were characterized by significantly higher total bilirubin values and higher AST, ALT, and ALB values compared to rats treated with CMFE. The most beneficial effect on the normalization of the above-mentioned parameters after MTX administration was CMFE treatment at a dose of 1400 mg·kg−1. | |||||
| [72][54] | Patients with non-alcoholic fatty liver disease (NAFLD) | 20 cc/d CMFE, 12 weeks | Placebo, 12 weeks | Treatment group, compared to the control group, showed a significant reduction in DBP and SBP. No difference between groups in weight, WC, HC, WHR, BFM, BFP, and FFM. Significant reduction in the treatment group compared to the control group in BFM and BFP. | Protective effect on the kidneys | [53][35] | |||
| [ | |||||||||
| Protective effect on the urinary system | Wistar strain male albino rats treated with carbon tetrachloride- | [73][55] | Prophylactic groups: CMFE 300 and 700 mg·kg−1, for 16 days, respectively and on the 16th day received CCl4 and curative groups: distilled water orally for 16 days and on the 16th day they received CCl4 (1 mL·kg−1 b.w.; 80% in olive oil), followed by CMFE 300 mg·kg−1 and 700 mg·kg−1, respectively, at 2, 6, 12, 24 and 48 h after CCl4 intoxication | “Sham” control for both preventive and therapeutic studies, receiving raw water and free access to food for 16 days and control for both preventive and therapeutic studies, receiving distilled water orally for 16 days, and on day 16 received olive oil daily (1 mL kg−1 | 55][37] | Wistar rats with streptozotocin-induced Alzheimer’s Disease | Flavonoid from CMFE at 5, 10 and 20 mg·kg−1 | saline-saline control, streptozotocine-saline control | CMFE treatment increased memory retention in a dose-dependent manner. The dose of 10 mg kg–1 decreased rat weight significantly. |
| Women with chronic cystitis (UTIs) | C. mas | tablet 500 mg·day | −1, 6 month | b.w.) | Different doses of fruit extract (300 and 700 mg/kg−1) significantly ameliorated the alterations induced by CCl4 in lipid peroxidation, antioxidant defenses, and biochemical and renal lesions. The level of antioxidant enzymes such as SOD, CAT, and GPx decreased in the CCl4-treated group and improved by treatment with CMFE. | ||||
| Placebo, 12 weeks | C. mas | decreases dysuria among patients with UTIs. | Protective effect on the brain | [54][36] | 12-week Wistar strain male albino rats | Rats with fructose diet (with CMFE) | Rats with a diet enriched in fat | Addition of CMFE stimulates PON activity, both in brain tissue and in plasma, and increases the protection of the nervous system from oxidative stress by increasing the activity of CAT. Protects proteins against peroxidation, as can be shown by the level of PCG. | |
| Protective effect on bones | [56][38] | Zucker diabetic fatty (ZDF) rats | diabetic obese rats receiving 500 and 1000 mg·kg−1 b.w. of CMFE |
non-diabetic lean rats | A higher dose of CMFE had a beneficial impact on femoral weight, cortical bone thickness, relative volume of trabecular bone, and trabecular thickness. |
||||
| [57][39] | 12-week-old female Wistar Rats with ovariectomy-induced metabolic changes |
ovariectomized animals receiving 17β-oestradiol; group with CMFE (50 mg·kg−1) |
“Sham” operated group and ovariectomized control group | CMFE ameliorated ovariectomy-induced decrease in femoral and tibial bone mineral density (BMD), prevented the deterioration in Young’s modulus and flexural strength and counteracted ovariectomy-induced decrease in serum calcium level. |
|||||
| Antihyperglycemic | [58][40] | Adult male rats with alloxan-induced diabetes | glibenclamide-treated (0.6 mg·kg−1·day−1; 4 weeks) and CMFE-treated (2 g−1 day; 4 weeks) group | non-diabetic control and diabetic control | Diabetic rats had significantly elevated levels of serum glucose, LDL-C, TG, AST, ALP, and ALT and decreased levels of HDL-C compared to the non-diabetic group. The effects of CMFE were comparable to those of glibenclamide at the doses tested in this study. | ||||
| [59][41] | Zucker diabetic fatty (ZDF) rats | CMFE in two doses (500 and 1000 mg·kg−1 b.w., 10 weeks) | non-diabetic lean controls received only distilled water | significant decrease of glucose level after oral administration of CMFE in a dose of 1000 mg/kg bw in the pre-diabetic state of animals (until the 7th week of the experiment) and significant restriction of water intake in both CMFE groups against the diabetic control. | |||||
| [25][5] | Male Wistar rats with streptozotocin-induced diabetes mellitus | CMFE extracts (20 mg kg−1 of b.w., 14 days) | control group (healthy) | CMFE lowered blood glucose and improved glucose tolerance. Significantly decreased the amount of glycated hemoglobin (by 25%) and increased erythrocyte resistance to acid hemolysis. | |||||
| Antihyperlipidemic effects | [60][42] | C57BL/6 mice fed a high-fat diet | mice were fed with a high-fat diet plus anthocyanins (1 g·kg−1) or ursolic acid 500 mg·kg−1) | mice were fed a normal diet | The anthocyanin showed a 24% decrease in weight gain and decreased lipid accumulation in the liver, including a significant decrease in liver triacylglycerol concentration. Anthocyanin and ursolic acid have extremely elevated insulin levels. | ||||
| [58][40] | Adult male rats with alloxan-induced diabetes mellitus | glibenclamide-treated (0.6 mg/kg/day; 4 weeks) and CMFE (2 g·day−1; 4 weeks) group | non-diabetic control and diabetic control, | Treatment with glibenclamide or CM counterbalanced significantly increased serum glucose, LDL-C, TG, AST, ALP, and ALT levels and decreased HDL-C levels in diabetic rats. | |||||
| [61,62,63][43][44][45] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | CMFE (100 mg·kg−1 b.w., 60 days) or simvastatin (5 mg·kg−1 b.w., 60 days) or loganic acid (20 mg·kg−1 b.w.) | control group with a standard diet and group with hyperlipidemia | CMFE led to a 44% significant decrease in serum TG levels and prevented the development of atheromatous changes in the thoracic aorta. CMFE significantly increased PPARα, had a significant protective effect on diet-induced oxidative stress in the liver, as well as restored upregulated pro-inflammatory cytokines serum levels. | |||||
| [64][46] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | CMFE (10 or 50 mg·kg−1 b.w.) or simvastatin (5 mg·kg−1 b.w.) | control group with a standard diet for rabbits and a group with hyperlipidemia | CMFE enhancement in PPAR-α and PPAR-γ expression in the aorta, LXR-α expression in the liver, a decrease in TG, leptin, and resistin, and an increase in adiponectin levels. | |||||
| [65][47] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | Group with a standard diet containing C. mas powder (1 g·kg−1 b.w.) diet, and with a high cholesterol (1%) containing C. mas powder (1 g·kg−1) diet, and a high cholesterol containing lovastatin (10 mg·kg−1 b.w.) diet | control group with a standard diet for rabbits and with a high cholesterol (1%) diet | C. mas powder and lovastatin significantly decreased fibrinogen levels in comparison with the high cholesterol group. Furthermore, C mas. powder could reduce the fibrinogen level more than lovastatin | |||||
| [66][48] | Healthy male Wistar rats | CMFE (50, 200 and 400 mg·kg−1 b.w., 3 weeks) | Normal control with normal diet without any injection and placebo control and intraperitoneally received a normal saline | All doses of CMFE significantly decreased HDW and PDW vs. the control group. Only high doses caused a significant elevation in MCHC, MPV, and PCT and a significant decrease in RDW vs. the control group. | |||||
| Protective effect on the eyes | [67][49] | Sexually mature, New Zealand white rabbits, aged between 6 and 12 months, were used: 7 males and 7 females | Intraconjunctival administration of one drop loganic acid or polyphenolic fraction of C. mas, corresponding to a volume of 50 µL, to the right eye | One drop of artificial tears containing 0.15% sodium hyaluronate (50 µL) was administered to the left eye as a placebo | Loganic acid (50%) and pelargonidin-3-galactoside (7%) were found as the main components of C. mas fraction. Therefore, the hypotensive effect was attributed to loganic acid. |
ALB—albumin, ALP—alkaline phosphatase, ALT—alanine amino transferase, AST—aspartate amino transferase, CAT—catalase, CMFE—Cornus mas fruit extract, GPx—glutathione peroxidase, HDL-C—high, density lipoprotein cholesterol, HDW—hemoglobin distribution width, LDL-C—low-density lipoprotein cholesterol, MCHC—mean corpuscular hemoglobin concentration, MDA—malondialdehyde, MPV—mean platelet volume, PCG—protein carbaryl groups, PCT—total platelet mass, PDW—platelet distribution width, PON—paraoxonase enzyme activity, RDW—red cell distribution width, SOD—superoxide dismutase, TG—triglycerides.
Table 3. Health-promoting properties of cornelian cherries anthocyanins proven in human studies, presented according to the PICO scheme.
AST—aspartate amino transferase, ALT—alanine amino transferase, BFM—body fat mass, BFP—body fat percent, DBP—diastolic blood pressure, FFM—fat-free mass HC—hip circumference, HDL-C—high, density lipoprotein cholesterol, ICAM-1—intracellular adhesion molecule-1, LDL-C—low-density lipoprotein cholesterol, NAFLD—non-alcoholic fatty liver disease, SBP—systolic blood pressure, VCAM-1—vascular cell adhesion molecule-1; WC—waist circumference, WHR—waist-to-hip ratio.
References
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit. Ornam. Plant Res. 2008, 68, 5–22.
- Klymenko, S. Phenological Stages of Development of Cornus L. S. Str. Species (Cornaceae) According to BBCH Scale. Agrobiodivers Improv. Nutr. Health Life Qual. 2021, 5, 185–196.
- Popović, B.M.; Štajner, D.; Slavko, K.; Sandra, B. Antioxidant Capacity of Cornelian Cherry (Cornus mas L.)—Comparison between Permanganate Reducing Antioxidant Capacity and Other Antioxidant Methods. Food Chem. 2012, 134, 734–741.
- Łysiak, G. Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods 2022, 11, 948.
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic Effects of Extracts of Red and Yellow Fruits of Cornelian Cherries (Cornus mas L.) on Rats with Streptozotocin-Induced Diabetes Mellitus. Food Funct. 2019, 10, 6459–6472.
- Chaves-Silva, S.; Santos, A.L.D.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the Genetic Regulation of Anthocyanin Biosynthesis in Plants—Tools for Breeding Purple Varieties of Fruits and Vegetables. Phytochemistry 2018, 153, 11–27.
- Borroto Fernández, E.G.; Mokhber, A.; Zeiser, M.; Laimer, M. Phenotypic Characterization of a Wild-Type Population of Cornelian Cherries (Cornus mas L.) from Austria. Erwerbs-Obstbau 2022, 64, 673–683.
- Martinović, A.; Cavoski, I. The Exploitation of Cornelian Cherry (Cornus mas L.) Cultivars and Genotypes from Montenegro as a Source of Natural Bioactive Compounds. Food Chem. 2020, 318, 126549.
- Milenkovic-Andjelkovic, A.; Radovanovic, B.; Andjelkovic, M.; Radovanovic, A.; Nikolic, V.; Randjelovic, V. The Anthocyanin Content and Bioactivity of Cornelian Cherry (Cornus mas) and Wild Blackberry (Rubus fruticosus): Fruit Extracts from the Vlasina Region. Adv. Tech. 2015, 4, 26–31.
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. Eur. Food Res. Technol. 2019, 245, 2071–2087.
- Antolak, H.; Czyzowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kregiel, D. Phenolic Compounds Contained in Little-Known Wild Fruits as Antiadhesive Agents Against the Beverage-Spoiling Bacteria Asaia spp. Molecules 2017, 22, 1256.
- Ochmian, I.; Oszmiański, J.; Lachowicz, S.; Krupa-Małkiewicz, M. Rootstock Effect on Physico-Chemical Properties and Content of Bioactive Compounds of Four Cultivars Cornelian Cherry Fruits. Sci. Hortic. 2019, 256, 108588.
- Sengul, M.; Eser, Z.; Ercisli, S. Chemical Properties and Antioxidant Capacity of Cornelian Cherry Genotypes Grown in Coruh Valley of Turkey. ASPHC 2014, 13, 73–82.
- Begic-Akagic, A.; Drkenda, P.; Vranac, A.; Orazem, P.; Hudina, M. Influence of Growing Region and Storage Time on Phenolic Profile of Cornelian Cherry Jam and Fruit. Eur. J. Hortic. Sci. 2013, 78, 30–39.
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; De Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 Involvement in Anthocyanin Gastric Transport- Nanobased Targeted Approach. Sci. Rep. 2019, 9, 789.
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The Interaction of Anthocyanins with Bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636.
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Small Intestine in Rats. J. Nutr. 2004, 134, 2275–2279.
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of Human Oral Mucosal Tissue, Saliva and Oral Microflora on Intraoral Metabolism and Bioactivation of Black Raspberry Anthocyanins. Cancer Prev. Res. 2011, 4, 1209.
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and Absorption of Red Grape and Wine Anthocyanins through the Gastrointestinal Tract. Trends Food Sci. Technol. 2019, 83, 211–224.
- Kalt, W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24, 4024.
- Keppler, K.; Humpf, H.-U. Metabolism of Anthocyanins and Their Phenolic Degradation Products by the Intestinal Microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205.
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114.
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, Quantification, and Bioactivities of Anthocyanins in Cornus Species. J. Agric. Food Chem. 2002, 50, 2519–2523.
- Yousefi, B.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti-Proliferative Properties of Cornus mass Fruit in Different Human Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5727–5731.
- Odžaković, B.; Sailović, P.; Bodroža, D.; Kojić, V.; Jakimov, D.; Kukrić, Z. Bioactive Components and Antioxidant, Antiproliferative, and Antihyperglycemic Activities of Wild Cornelian Cherry (Cornus mas L.). Maced. J. Chem. Chem. Eng. 2021, 40, 221.
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53, 28–31.
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In Vitro α-Amylase and Pancreatic Lipase Inhibitory Activity of Cornus mas L. and Cornus alba L. Fruit Extracts. J. Food Drug Anal. 2019, 27, 249–258.
- Zarei, L.; Sadrkhanlou, R.; Shahrooz, R.; Malekinejad, H.; Eilkhanizadeh, B.; Ahmadi, A. Protective Effects of Vitamin E and Cornus mas Fruit Extract on Methotrexate-Induced Cytotoxicity in Sperms of Adult Mice. Vet. Res. Forum 2014, 5, 21–27.
- Eshaghi, M.; Zare, S.; Banihabib, N.; Nejati, V.; Farokhi, F.; Mikaili, P. Cardioprotective Effect of Cornus mas Fruit Extract against Carbon Tetrachloride Induced-Cardiotoxicity in Albino Rats. J. Basic Appl. Sci. Res. 2012, 2, 11106–11114.
- Moayed Alavian, S.; Banihabib, N.; Es. Haghi, M.; Panahi, F. Protective Effect of Cornus mas Fruits Extract on Serum Biomarkers in CCl4-Induced Hepatotoxicity in Male Rats. Hepat. Mon. 2014, 14, e10330.
- Somi, M.H.; Banihabib, N.; Dehghan, G.; Es. Haghi, M.; Panahi, F. Hepatoprotective Effect of Cornus mas Fruits Extract Against Carbon Tetrachloride-Induced Hepatic Damage in Male Albino Rats. Thrita 2014, 3, e17625.
- Saei, H.; Hatami, H.; Azarmi, M.; Dehghan, G. Hepatoprotective Effect of Cornus mas Fruit Extract on Serum Biomarkers in Methotrexate-Induces Liver Injury in Male Rats. Pharmacol. Line 2016, 1, 91–98.
- Es Hagi, M.; Dehghan, G.; Banihabib, N.; Zare, S.; Mikalili, P.; Panahi, F. Protective Effects of Cornus mas Fruit Extract on Carbon Tetrachloride Induced Nephrotoxicity in Rats. Indian. J. Nephrol. 2014, 24, 291.
- Francik, R.; Kryczyk, J.; Krośniak, M.; Berköz, M.; Sanocka, I.; Francik, S. The Neuroprotective Effect of Cornus mas on Brain Tissue of Wistar Rats. Sci. World J. 2014, 2014, 847368.
- Darbandi, N.; Hashemi, A.; Noori, M.; Momeni, H.R. Effect of Cornus mas Fruit Flavonoids on Memory Retention, Level of Plasma Glucose and Lipids in an Intracerebroventricular Streptozotocin-Induced Experimental Alzheimer’s Disease Model in Wistar Rats. EEB 2016, 14, 113–120.
- Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals 2020, 10, 2435.
- Nowak, B.; Matuszewska, A.; Szeląg, A.; Danielewski, M.; Dziewiszek, W.; Nikodem, A.; Filipiak, J.; Jędrzejuk, D.; Bolanowski, M.; Kucharska, A.Z.; et al. Cornelian cherry (Cornus mas L.) Extract Reduces Cardiovascular Risk and Prevents Bone Loss in Ovariectomized Wistar Rats. J. Funct. Foods 2022, 90, 104974.
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and Histopathological Study of the Anti-Hyperglycemic and Anti-Hyperlipidemic Effects of Cornelian cherry (Cornus mas L.) in Alloxan-Induced Diabetic Rats. J. Complement. Integr. Med. 2014, 11, 63–69.
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Svik, K.; Prnova, M.S.; Slovak, L.; Kovacik, A.; Lory, V.; Zorad, S.; et al. Cornelian Cherry Fruit Improves Glycaemia and Manifestations of Diabetes in Obese Zucker Diabetic Fatty Rats. Res. Vet. Sci. 2019, 126, 118–123.
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248.
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The Protective Effect of the Cornus mas Fruits (Cornelian cherry) on Hypertriglyceridemia and Atherosclerosis through PPARα Activation in Hypercholesterolemic Rabbits. Phytomedicine 2014, 21, 1774–1784.
- Sozański, T.; Kucharska, A.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic Acid and Anthocyanins from Cornelian cherry (Cornus mas L.) Fruits Modulate Diet-Induced Atherosclerosis and Redox Status in Rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513.
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–Loganic Acid versus Anthocyanins from the Cornus mas Fruits (Cornelian cherry): Common and Different Effects on Diet-Induced Atherosclerosis, PPARs Expression and Inflammation. Atherosclerosis 2016, 254, 151–160.
- Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomułkiewicz, A.; Dzimira, S.; Dzięgiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients 2021, 13, 3621.
- Asgary, S.; Rafieian-Kopaei, M.; Adelnia, A.; Kazemi, S.; Shamsi, F. Comparing the Effects of Lovastatin and Cornus mas Fruit on Fibrinogen Level in Hypercholesterolemic Rabbits. ARYA Atheroscler. 2010, 6, 1–5.
- Abdollahi, B.; Mesgari Abbasi, M.; Zakeri Milani, P.; Sadat Nourdadgar, A.; Banan Khojasteh, S.M.; Nejati, V. Hydro-Methanolic Extract of Cornus mas L. and Blood Glucose, Lipid Profile and Hematological Parameters of Male Rats. Iran. Red. Crescent Med. J. 2014, 16, e17784.
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid.-Based Complement. Altern. Med. 2015, 2015, 939402.
- Sangsefidi, Z.S.; Hosseinzadeh, M.; Ranjbar, A.M.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The Effect of Total Anthocyanin-Base Standardized (Cornus mas L.) Fruit Extract on Liver Function, Tumor Necrosis Factor α, Malondealdehyde, and Adiponectin in Patients with Non-Alcoholic Fatty Liver: A Study Protocol for a Double-Blind Randomized Clinical Trial. Nutr. J. 2019, 18, 39.
- Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Ranjbar, A.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The Effect of (Cornus mas L.) Fruit Extract on Liver Function among Patients with Nonalcoholic Fatty Liver: A Double-blind Randomized Clinical Trial. Phytother. Res. 2021, 35, 5259–5268.
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the Effects of Cornus mas L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 740954.
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the Lipid-Modifying and Antiinflammatory Effects of Cornus mas L. Supplementation on Dyslipidemic Children and Adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735.
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. Fruit Extract on Blood Pressure, Anthropometric and Body Composition Indices in Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 56, 18–24.
- Dadkhah, N.; Shirani, M.; Etemadifar, S.; Mirtalebi, M. The Effect of Cornus mas in Preventing Recurrent Urinary Tract Infections in Women. Future Nat. Prod. 2017, 3, 67–76.
More
