One of the main challenges in treating GBM is the highly complex and dynamic nature of the GBM tumour microenvironment, which plays a crucial role in tumour growth, invasion, and resistance to therapy
[5]. The GBM tumour microenvironment comprises various cell types, including tumour cells, astrocytes, microglia, endothelial cells, and immune cells, as well as extracellular matrix components, growth factors, and cytokines
[6][7][6,7]. The interactions between these components create a highly heterogeneous and dynamic environment that facilitates tumour progression and adaptation to therapy
[8]. Recent studies have highlighted the importance of the macroenvironment and microbiome in GBM pathogenesis and treatment
[9][10][9,10]. Tumours release factors that drive the orchestration of an environment in the host that involves the crosstalk between multiple distal compartments at places beyond tumour beds
[11]. Systemic alterations include changes in the bone marrow’s functioning, where myelopoiesis is especially heavily altered in the presence of a tumour
[12][13][12,13]. Distal hormonal signals and inflammatory mediators generated through interactions with commensal microorganisms also facilitate the formation of premetastatic niches where disseminated tumour cells call home, lay dormant, and eventually develop into growing metastatic
[14][15][16][17][14,15,16,17]. Together, these inflammatory, tumour-promoting pro-metastatic networks form a systemic “macroenvironment” in tumour-bearing hosts that influence distant tissues' function and the tumour itself
[18]. In the current era of personalised medicine, identifying and comprehensively understanding cancer’s pathophysiological mechanisms are crucial for tailoring therapies based on grade, histological features, molecular subtypes, aggressiveness, and treatment response.
The endocannabinoid system (ECS) is a widespread neuromodulatory network that plays a role in the development and maturation of nervous systems by modulating network function and neuronal activity
[19]. G-protein coupled cannabinoid receptors including the canonical receptor subtypes cannabinoid receptor type 1 (CB1-R) and cannabinoid receptor type 2 (CB2-R), endogenous cannabinoids known as endocannabinoids (e.g., anandamide and 2-arachidonoylglycerol), and the proteins that synthesize and degrade endocannabinoids, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), comprise the endocannabinoid system
[20][21][20,21]. In addition to the enzymes involved in the biosynthesis and degradation of endocannabinoids, the other “non-canonical” extended signalling network of the ECS include receptors GPR55 and PPARα, inotropic cannabinoid receptors (TRP channels), protein transporters (FABP family), and other fatty acid derivatives
[22][23][24][25][22,23,24,25]. While cannabinoid receptors are present in most tissues, CB1-R is primarily and found mainly in the CNS, moderately found in adipose, endocrine, lymphoid, and female tissues and in smaller amounts found in other tissues
[26]. The endocannabinoid system has emerged as a potential target for treating GBM
[27]. The ECS is a complex signalling system that plays a crucial role in maintaining homeostasis in the body
[28]. The ECS involves various physiological processes, including pain modulation, appetite, mood, and immune function
[29]. Recent evidence suggests that the ECS is dysregulated in GBM and that its manipulation could represent a promising therapeutic strategy
[30][31][30,31]. In particular, CB1-R and CB2-R are expressed in GBM cells and the tumour microenvironment, including immune cells and endothelial cells
[32][33][34][32,33,34]. Activation of these receptors has been shown to induce antitumour effects in preclinical studies, including inhibition of tumour cell proliferation, migration, invasion, and angiogenesis
[35].
2. Cannabinoids as a Promising Adjuvant in the Treatment of GBM
The endocannabinoid system which includes endocannabinoids and the enzymes that synthesise and degrade them, and the transporters and G-protein coupled receptors involved in their signalling have been found in glioblastoma cells
[31][40][31,101]. The ECS is a homeostatic system that uses lipid-derived signalling molecules to regulate a wide range of physiological functions
[41][102]. Studies have shown high levels of cannabinoid receptors, CB1-R and CB2-R, as well as the transient receptor potential vanilloid 1 receptor expressed on glioblastoma cells, which are regulated by genetic and epigenetic mechanisms
[42][103]. Although the expression levels obtained by immunohistochemistry are heterogeneous and dependent on the age of the patient and the histopathological origin of the brain tumour cells, CB2-R expression has been positively correlated with tumour grade and upregulated in most glioblastomas
[43][104]. According to immunohistochemical analysis, both CB1 and CB2 receptors were detected in around 38% and 54% of glioblastoma endothelial cells, respectively
[44][105]. CB2-R expression levels were found to be higher than CB1 in glioblastoma tissues. These findings suggest that selective CB2-R agonists could potentially serve as crucial targets for the treatment of glioma. The term “cannabinoids” originally described bioactive constituents of the
Cannabis sativa plant. It is now an umbrella term covering a broad range of compounds subsectioned into the synthetic cannabinoids, the phytocannabinoids, and the endogenous cannabinoids, most of which are ligands which bind to endogenous cannabinoid (e.g., CB1-R and CB2-R) and other G-protein coupled receptors
[44][45][105,106]. The endogenous cannabinoids are naturally occurring lipid mediators that are synthesised from the membrane phospholipids of cells
[46][107].
Table 14 provides an overview of the main classes of cannabinoids: classical cannabinoids, non-classical cannabinoids, aminoalkylindoles, and eicosanoids
[47][108]. The table summarises the structural characteristics, formulation strategies, and metabolism for each class. This information can be used to understand the unique properties of each class of cannabinoids.
Table 14.
Cannabinoids: A Classification Based on Structural Features and Pharmacological Effects.
Cannabinoid receptor activation can lead to the modulation of downstream signalling pathways in glioblastoma cells, including the PI3K/Akt/mTOR pathway, the MAPK/ERK pathway, and the c-Jun N-terminal kinase (JNK) pathway
[62][63][123,124]. The activation of these pathways can have diverse effects on cell proliferation, differentiation, survival, and migration, depending on the specific context and the balance of signalling inputs. In addition to the modulation of signalling pathways, cannabinoids can also regulate gene expression in glioblastoma cells. For example, some cannabinoids, such as THC, have the capacity to influence the expression of the tumour suppressor gene for p53
[64][125], while inhibiting the expression of genes involved in cell cycle progression and angiogenesis, such as cyclin A and D1 and VEGF
[65][66][67][68][126,127,128,129]. The molecular mechanisms of cannabinoid action in glioblastoma are complex and involve both receptor-dependent and -independent pathways. In addition to the modulation of the ECS and downstream signalling pathways, cannabinoids can also interact with other targets, such as ion channels, other G protein-coupled receptors, and nuclear receptors
[69][70][71][72][130,131,132,133]. An increasing number of preclinical models and clinical studies have investigated the anti-cancer effects of cannabinoids on a variety of cancers
[73][134]. Reports have shown a dysregulation of cannabinoid receptors and endogenous ligands present in the tumour microenvironment of cancerous tumours; however, the ‘endocannabinoid’s system role suggests both pro-tumourigenic and anti-cancer effects based on the type and site of cancer
[74][135]. Some authors attribute these inconsistencies to an incomplete elucidation of this complex biological system, the bystander effect or the heterogeneity of receptors present in the disease state
[42][103]. An important systematic review that the 2017 National Academy of Sciences committee used to review the health effects of cannabis-focused on gliomas and identified 2260 studies, of which 35 met the inclusion criteria
[75][76][136,137]. Sixteen of these studies were in vivo studies which described the anti-cancer effects of cannabinoids on glioma tumours
[31]. Meanwhile, many
in vitro and preclinical studies in animal models have successfully shown the anti-cancer effects of cannabinoids based on the reduction of tumour growth via the inhibition of tumour cell proliferation and angiogenesis, the tumour microenvironment, induction of tumour cell death, and inhibition of invasion through the genetic or pharmacological modulation of cannabinoid and other receptors
[68][77][78][79][80][81][129,138,139,140,141,142]. A study assessing the need for the addition of serum to
in vitro testing conditions of cannabinoids reaffirmed the importance of mimicking the tumour microenvironment in vitro and warned about the high degree to which cannabinoids bind to plastic
in vitro [82][143]. This is because the tumour microenvironment is a complex and dynamic environment that can influence the efficacy of cannabinoids. By mimicking the tumour microenvironment
in vitro, researchers can develop more accurate and predictive models of cannabinoid activity
[83][144]. This can help to prevent clinical failure associated with differences between
in vitro models and human subjects. A study investigated the
in vitro and
in vivo efficacy of cannabidiol (CBD) in neuroblastoma, a nervous system tumour in children
[84][145]. Two cannabinoids, tetrahydrocannabinol (THC) and CBD were experimentally tested to determine the effects of the compounds on invasiveness, programmed cell death, viability, and cell cycle distribution in human neuroblastoma cells
in vitro. The cannabinoids were also evaluated for their ability to reduce the growth of tumour xenografts
in vivo in mice. The results showed that both THC and CBD had antitumourigenic activity
in vitro. However, CBD was more active than THC in reducing the invasiveness, apoptosis, viability, and cell cycle distribution of neuroblastoma cells.
In vivo, CBD also showed greater efficacy than THC in reducing the growth of tumour xenografts in mice. Further studies are needed to confirm these findings and to evaluate the safety and efficacy of CBD in clinical trials.
The levels of endocannabinoids and expression of their receptors present in the glioblastoma microenvironment are dysregulated in the disease state and this dysregulation is thought to contribute to the growth and progression of GBM tumours
[27].
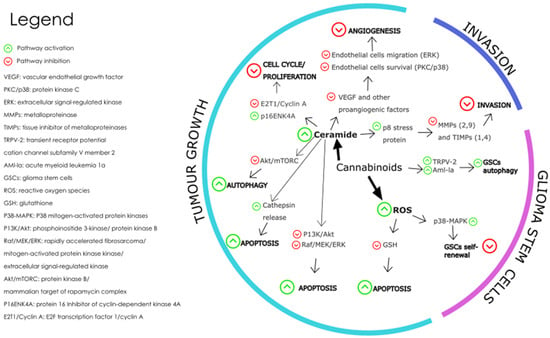
Figure 15 shows the pathways triggered by cannabinoid receptor interaction, which affect the hallmarks of cancer associated with glioblastoma tumours.
Figure 15. The main molecular mechanisms affected by cannabinoids during the modulation of GBM are depicted where the green arrows represent pathway activation, and the red arrows represent pathway inhibition
[85][146]. Redrawn with permission from Dumitru, C.A., Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs; published by Front Mol Neurosci, 2018. Creative Commons CC BY 4.0.
http://creativecommons.org/licenses/by/4.0/ (accessed on 26 June 2023).
The mechanisms involved in the effect of cannabinoids on GBM tumour growth include cell death-inducing mechanisms, anti-angiogenic mechanisms, and anti-proliferation mechanisms. Cannabinoid-induced cell death is prompted by the activation of the intrinsic apoptosis pathway by cannabinoid-receptor interaction, which results in increased intracellular ceramide, thereby inhibiting pathways PI3K/Akt and Raf1/MEK/ERK
[63][124]. The PI3K/Akt/mTOR pathway is a key signalling pathway that regulates cell proliferation, survival, and metabolism. Activation of CB1 and CB2 receptors by cannabinoids has been shown to inhibit the PI3K/Akt/mTOR pathway in glioblastoma cells, leading to a decrease in cell proliferation and an increase in apoptosis and autophagy
[86][147]. This effect is thought to be mediated by the inhibition of Akt phosphorylation and activation and the downregulation of downstream targets such as mTOR, p70S6K, and 4EBP1
[87][148].
Cannabinoid-induced apoptosis is also triggered by oxidative stress, as seen when glioma cells treated with CBD caused an increase in reactive oxygen species (ROS) formation
[88][89][149,150]. The MAPK/ERK pathway is another important signalling pathway that regulates cell proliferation, differentiation, and survival. Activation of CB1 and CB2 receptors by cannabinoid ligands has been shown to modulate the MAPK/ERK pathway in glioblastoma cells, leading to a decrease in angiogenesis and an increase in apoptosis
[90][91][151,152]. This effect is thought to be mediated by the inhibition of ERK phosphorylation and activation and the downregulation of downstream targets such as c-fos and c-jun.
In a recent study, a standard mix of
cannabis-extracted active fractions F4 and F5 was found to induce apoptosis and expression of endoplasmic reticulum (ER)-stress-associated genes in glioblastoma cells
[92][153]. The fractions F4 and F5 also inhibited cell migration and invasion, altered cell cytoskeletons, and inhibited colony formation in 2 and 3-dimensional models. The study suggests that combinations of cannabis compounds exert cytotoxic, anti-proliferative, and anti-migratory effects on glioblastoma cells. The JNK pathway is a stress-activated signalling pathway that regulates cell survival and apoptosis
[93][154]. Activation of CB1 and CB2 receptors by cannabinoids has been shown to activate the JNK pathway in glioblastoma cells, leading to increased apoptosis
[94][155]. This effect is thought to be mediated by the activation of JNK phosphorylation and the upregulation of downstream targets such as c-jun. Further research is needed to fully understand the molecular mechanisms of cannabinoid action in glioblastoma, as well as the potential for developing cannabinoid-based therapies for this deadly disease
[95][156].
The physicochemical properties of most traditional cannabinoids, which include high lipophilicity, poor water solubility, and chemical instability, present significant formulation challenges for the development of effective therapies for brain tumours. However, advances in pharmaceutical science and technology are helping to overcome these challenges and to harness the potential of cannabinoids for the treatment of brain tumours. The lipophilic nature of cannabinoids may be beneficial for cannabinoid delivery to the brain but tend to lend to the formation of colloidal aggregates, which artefacts in early drug discovery and proves difficult to achieve suitable solubility and stability in aqueous solutions. However, they possess an attractive composition as nanoparticle formulations for targeted drug delivery. A combination of ligand proteins and polymers may be used to stabilise the colloidal aggregates, reduce colloid size, and improve longevity in blood circulation
[96][157]. Besides the high hydrophobicity associated with most cannabinoids, including THC, the ability to elicit CB1-R mediated psychoactivity is one of the most noted drawbacks of cannabinoid therapeutic use
[97][98][158,159]. All sources of evidence investigated in a recent study, including randomised controlled trials, observational studies, and Mendelian Randomisation studies, have consistently indicated the use of cannabis is associated with an increased risk of psychosis and a potentially increased risk of psychiatric symptoms such as mania
[99][100][160,161]. A systematic review of the safety of cannabinoids for medical use was conducted
[101][162]. There is insufficient data on the safety of cannabinoids, but most studies reported no adverse events (AEs) with acute administration and mild to moderate AEs with chronic administration. The most common AEs reported were drowsiness, fatigue, and dry mouth
[102][163]. An association between cognitive impairment and cannabis has been shown in observational studies and randomized controlled trials, which have also been associated with motor vehicle accidents
[103][164]. While CBD has demonstrated promising efficacy in various clinical trials, it is essential to recognize its intrinsic pharmacological effects, potential adverse drug events, and the possibility of pharmacokinetic and pharmacodynamic drug-drug interactions
[104][165]. Given the increasing prevalence of CBD use among patients with complex medical conditions and treatment regimens, as well as its widespread availability as a consumer product, a comprehensive understanding of CBD’s safety profile is paramount
[105][166]. Further research is needed to better understand the safety of cannabinoids for medical use.