Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chao-Liang Wu and Version 2 by Rita Xu.
Alterations in the activity of BK
Ca
channels, responsible for the generation of the overall magnitude of Ca
2+
-activated K
+
current at the whole-cell level, occur through allosteric mechanisms. The collaborative interplay between membrane depolarization and heightened intracellular Ca
2+
ion concentrations collectively contribute to the activation of BK
Ca
channels.
- large-conductance Ca2+-activated K+ channel
- Ca2+-activated K+ current
- membrane potential
- cardiac action potential
1. The Role of BKCa Channel Activity Residing in Cardiac Fibroblasts
Fibroblasts, residing in connective tissue, are pivotal for synthesizing the extracellular matrix and collagen, thereby offering structural support to tissues, and playing a critical role in the process of wound healing. Specifically, cardiac fibroblasts, located within the heart tissue, are responsible for both the synthesis and maintenance of the extracellular matrix, which is indispensable for providing essential structural support to the heart. This matrix serves as a foundational framework for cardiomyocytes, the muscle cells responsible for the heart’s contraction.
Unlike cardiac muscle cells (cardiomyocytes), which are responsible for rhythmic and coordinated contraction, cardiac fibroblasts, classified as connective tissue cells, are indispensable for upholding the structural integrity of the heart. This is achieved through the synthesis of collagen and other proteins located within the myocardial tissue of the heart. Functional integrity of the heart tissue pertains to the overall health, stability, and proper operation of the various components comprising the physical structure of the heart. The well-developed heart is a complex organ composed of different types of cells, including cardiomyocytes, fibroblasts, blood vessels, and extracellular matrix.
BKCa channels undergo substantial activation in response to either depolarization of the cell membrane or an increase in intracellular Ca2+ levels. Furthermore, the whole-cell IK(Ca), where BKCa channel activity is relevant, displays a pronounced outward rectifying characteristic [1][2][3][3,13,16]. In the context of KV channels, outward rectification means that the channel primarily allows ions to move out of the cell in response to changes in membrane potential. In other words, the BKCa-channel’s conductance is higher for K+ ions moving in the outward direction (from inside the cell to the extracellular space) compared to K+ ions moving in the inward direction (from outside the cell to the intracellular space). This characteristic is significant in functions such as membrane hyperpolarization or repolarization, shaping action potentials, and the modulation of smooth muscle contraction [1][4][5][2,3,17].
However, owing to the outward rectifying property of BKCa channels, their functionality is relatively minimal when the cell membrane is at its resting potential. Consequently, in non-excitable cells such as fibroblasts, where changes in the cell membrane potential are minimal, the impact of BKCa channels on these cells seems to be constrained. In other words, if cardiac fibroblasts do not generate a heart-like action potential, their resting membrane potential is supposed to be roughly between −20 and −40 mV. In this case, BKCa channel activity is very weak. However, through effective electrical coupling, fibroblasts transition to having action potentials, causing the membrane potential to depolarize and consequently activate more BKCa channels. As a result, the magnitude of the whole-cell Ca2+-activated K+ current in cardiac fibroblasts increases significantly.
The action potential in the ventricles of mammalian hearts, excluding rodents, typically manifests as a square- or dome-shaped waveform, characterized by the presence of a plateau potential. The ventricular action potential refers to the sequence of electrical events that occur in the cardiac ventricular cells during each heartbeat. This process involves a series of phases, each characterized by specific changes in membrane potential. The interplay of specific ionic currents during the ventricular action potential ensures the proper coordination of electrical events leading to contraction and relaxation of the ventricular tissues.
It is worth noting, however, that a mitochondrial BKCa channel has been previously identified in dermal fibroblasts and heart cells [6][7][8][15,18,19]. These channels are located within the inner mitochondrial membrane, where they contribute to the regulation of mitochondrial function and cellular bioenergetics [9][10][10,20].
BKCa channel activity has been observed to contribute to membrane hyperpolarization in vascular endothelial cells, as part of endothelium-derived processes [9][10][10,20]. It is important to note that, unlike vascular smooth muscle cells, where the BKCa channels employ a negative feedback mechanism to regulate the excessive increase of intracellular Ca2+ ions, in vascular endothelial cells, the functioning of BKCa channels in vascular endothelial cells relies on positive feedback mechanisms to control the intracellular elevation of Ca2+ ions. Much like vascular or cardiac fibroblasts, a significant portion of vascular endothelial cells do not exhibit electrical excitability. Consequently, the control of intracellular Ca2+ within these cells relies on an electrochemical driving force, with a particular emphasis on the flux of Ca2+ ions. In simpler terms, the voltage gradient and concentration gradient of Ca2+ both align inward in these cells. The main reason for this is that the concentration of Ca2+ outside the cell is a thousand times greater than inside the cell, and Ca2+ itself carries a double positive charge. The electrochemical driving force refers to the combined influence of both the electrical gradient and the chemical (concentration) gradient acting on ions across a cell membrane. Resting intracellular Ca2+ concentrations in fibroblasts or endothelial cells are typically maintained at low levels within the cytoplasm, usually around 100 nM. The concentration of extracellular Ca2+ usually ranges from approximately 1.1 to 1.3 mM in the blood plasma. However, in response to stimulation, such as exposure to diverse signaling molecules or mechanical forces, a swift and transient elevation in intracellular Ca2+ levels can occur. Furthermore, the activation of BKCa channels is modulated by localized microdomains beneath the surface membrane. The precise intracellular concentration of Ca2+ concentration necessary for BKCa channel activation may vary contingent on the specific tissue or cell type.
However, in electrically excitable cells, such as vascular smooth muscle cells, a negative feedback mechanism operates, revealing a complex interplay during membrane depolarization. When membrane depolarization induces voltage-gated Ca2+ currents—such as T- or L-type Ca2+ currents—across the cell membrane, extracellular Ca2+ ions can readily ingress the cell. Concurrently, membrane depolarization activates voltage-gated Na+ current, further promoting cell depolarization. This dual effect, characterized by both membrane depolarization and elevated cytosolic Ca2+ concentrations, triggers the activation of BKCa channels. Consequently, these channels lead to membrane hyperpolarization by facilitating the efflux of K+ ions out of the cell. This hyperpolarization, in turn, results in the inactivation of voltage-gated Na+ and Ca2+ currents [1][4][11][2,3,21]. The activation of BKCa channels thus contributes to a retardation in the elevation of intracellular Ca2+, ensuring meticulous regulatory control.
The hERG or Kv7.1 channels indeed play a crucial role in the repolarization of the cardiac action potential. However, in mature cardiomyocytes, BKCa channel activity is absent. Consequently, how the activity of BKCa channels compares with other K+ channels in influencing the repolarization of mature cardiomyocytes remains unknown. It may be necessary to investigate whether effective electrical coupling, both qualitatively and quantitatively, can occur between these cardiac cells and the surrounding fibroblasts to gain insights into this aspect.
A noteworthy finding highlights the existence of BKCa channels in cardiac fibroblasts [2][12][13][13,14,22]. These channels are thought to play a role in facilitating potential electrical coupling between myocyte and fibroblast. Electrical coupling denotes the direct electrical link between neighboring cells, and computational modeling studies in silico lend support to this phenomenon [2][14][13,23] (Figure 1). This coupling is often used in the context of neurons or certain types of muscle cells where rapid and synchronized communication is essential. When cardiac fibroblasts are numerous and establish robust connections with neighboring cardiac cells through gap junctions or intercalated discs, functional electrical coupling can be ensured. Gap junctions, protein channels spanning the cell membranes of adjacent cardiomyocytes, are thought to allow for the direct exchange of ions (such as Na+, K+, and Ca2+ ions) and small molecules between neighboring cells [15][24]. Connexins are a family of proteins that play a critical role in the formation of gap junctions, specialized intercellular channels that allow direct communication between adjacent cells. These gap junctions enable the exchange of ions, small molecules, and signaling molecules, contributing to the coordination of physiological functions in tissues.
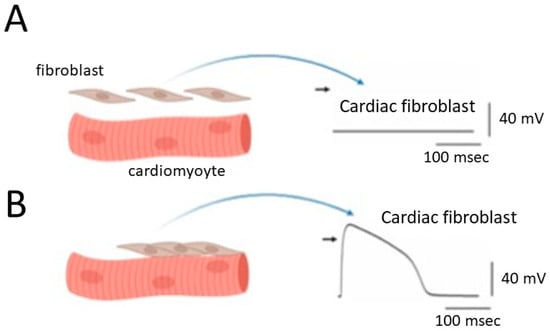
Figure 1. Alterations in the membrane potential of cardiac fibroblasts when they are structurally and electrically coupled to neighboring cardiomyocytes. Panel (A) represents this scenario in the absence of coupling, while panel (B) shows the situation in the presence of effective structural and electrical coupling. The graph depicts cardiac myocytes on the left side, showcasing evident striations. Importantly, the diagram highlights cardiac cells characterized by distinct striations, serving as an indicator of ventricular cells within the heart. Additionally, a comparable coupling phenomenon is observed in cardiac atrial cells when a sufficient number of large fibroblasts are present in the atrium. The black short arrow on the right side of (A,B) indicates the zero potential level.
Moreover, the intercalated discs in heart tissues are specialized structures found in the heart tissue that play a crucial role in facilitating communication and coordination between adjacent cardiac muscle cells of the heart. This can allow them to work together as a functional unit during the contraction and relaxation of the heart. These discs play a crucial role in maintaining the integrity of the cardiac tissue, ensuring the synchronization of both electrical signals and mechanical forces. This synchronization is essential for the efficient and coordinated contraction of the heart. In other words, cardiomyocytes and fibroblasts may form a functional syncytium, allowing for coordinated contraction and efficient pumping of blood. The functional syncytium pertains to the unified and synchronized contraction of cardiac muscle cells in the heart tissue. The ability of these cells to form a functional syncytium is facilitated by their electrical connectivity through gap junctions.
In this context, cardiac fibroblasts may display a “dome-like configuration” in their action potentials, referencing the unique shape observed in specific cardiac cell types, particularly ventricular cardiomyocytes [2][16][17][18][13,25,26,27] (Figure 1).
Consequently, the depolarization of fibroblasts becomes more pronounced, emphasizing the crucial role of BKCa channels in cardiac fibroblasts due to their outwardly rectifying property. This prompts an intriguing hypothesis that cardiac fibroblasts may engage in significant interactions with cardiomyocytes, potentially making a significant contribution to the heart’s overall structural and functional integrity (Figure 1). This hypothesis becomes especially pertinent when contemplating the potential augmentation of their numbers or sizes within cardiac tissues, particularly in conditions such as atrial fibrillation or preceding myocardial infarction [13][19][20][21][22][23][22,28,29,30,31,32]. Atrial fibrillation is a common and often chronic heart rhythm disorder that affects the upper chambers of the heart, known as the atria. In atrial fibrillation, the normal coordinated electrical impulses that regulate the heart’s rhythm become chaotic and irregular. Consequently, instead of the atria contracting efficiently to move blood into the ventricles, they quiver or fibrillate. The preceding or old myocardial infarction, commonly referred to as an “old heart attack”, is a term used to describe a previous heart attack or myocardial infarction that occurred in the past, and the affected tissue has undergone certain changes over time.
2. Influence of BKCa Channel Activity in Cardiac Fibroblasts on Membrane Potential of Heart Cells
Mammalian heart cells display a diverse cellular composition, placing primary emphasis on cardiomyocytes, cardiac fibroblasts, and endothelial cells. Notably, among the non-myocytes, cardiac fibroblasts are not electrically excitable and may constitute a substantial proportion, particularly in cases of atrial fibrillation or old myocardial infarction. These cardiac fibroblasts have been suggested to play crucial roles in shaping both the structure and functionality of the myocardium [17][20][26,29]. It is believed that these fibroblasts contribute to various aspects of cardiac performance, encompassing structural, biochemical, mechanical, and even electrical dimensions [16][17][20][25,26,29].
Although fully mature mammalian heart cells display electrical excitability, they do not possess functional activation of BKCa channels, despite the consistent functional expression of voltage-gated Na+ and Ca2+ currents in cardiac cells. Conversely, undifferentiated cardiomyocytes derived from embryonic stem cells have been observed to express the activity of these channels which is sensitive to be increased by membrane depolarization and/or elevated cytosolic Ca+ concentration [24][25][33,34]. The function of these channels in undifferentiated cardiomyocytes is also responsive to inhibition by paxilline, a natural product and a class of indole alkaloids known to effectively suppress the activity BKCa channels, but not by apamin, a blocker of small-conductance Ca2+-activated K+ channels. A contributing factor to this difference is the relatively prolonged duration of action potentials with a plateau potential in mature heart cells, which is attributed to the absence of functionally active BKCa channels [4][26][1,2]. Likewise, cardiomyocytes differentiated in vivo from amniotic fluid-derived stem cells showed no significant activity of BKCa cells [24][33]. Alternatively, earlier studies have suggested the possible presence of KCa1.1 (or KCNMA1) in human sinus node function [27][28][35,36].
Prior studies have shown that in cell-attached current-clamp voltage recordings, the sporadic opening and closure of BKCa channels were robustly observed in cardiac fibroblasts. The activity of these channels has the potential to induce random depolarizing waveforms via effective electrical coupling [24][29][30][33,37,38] (Figure 2). This phenomenon was also previously found in bovine chromaffin cells [31][39]. On the other hand, when heart cells are near cardiac fibroblasts, the activity of BKCa channels present on the surface of these fibroblasts can lead to varying degrees of membrane depolarization. When the cell membranes of these cardiac cells undergo random depolarization, coupled with the accumulation of temporal and spatial phenomena, it is likely to significantly increase the excitability of the cell membrane, even inducing the generation of action potentials. If the BKCa channels on cardiac fibroblasts are activated due to the treatment with the openers of the channel [3][10][12][32][11,14,16,20], it might even result in the facilitation of subtle membrane depolarization in neighboring heart cells. This effect is attributed to effective electrical coupling between cardiac fibroblasts and neighboring heart cells.
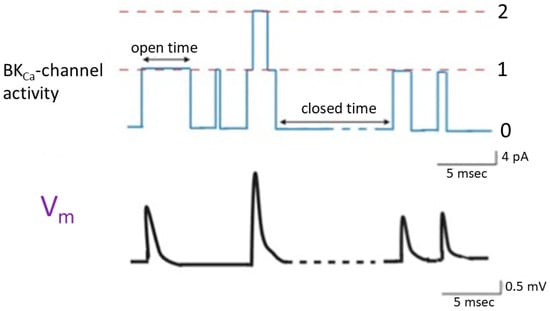
Figure 2. Changes in membrane potential (lower panel) triggered by the random opening of idealized BKCa channels (upper panel) inherently in cardiac fibroblasts. The simultaneous cell-attached potential and current recordings were made in this study, and cells were immersed in normal Tyrode’s solution which contained 5.4 mM K+, and 1.8 mM Ca2+. Tyrode’s solution is a physiological salt solution used in biological and medical research to mimic the extracellular fluid environment in which cells are studied. The dashed orange line in the upper panel represents each level of BKCa-channel opening, while the numerical values on the right indicate the number of channel openings. The double arrow in the channel activity indicates variable duration in the opening or closed state. The upper panel represents the activity of BKCa channels. The lower panel shows the changes in membrane potential induced by BKCa-channel activity. Upward deflection in current (blue color) and potential (black color) traces represents the occurrence of channel opening and membrane depolarization, respectively. In the bottom right corner of both the upper and lower panels, there are calibration marks. Of note, the occurrence of a transient depolarization is not triggered by the second channel opening event, as this event exhibits a brief duration of channel openness, which is less than 1 msec.
