Obesity is a prevalent health condition associated with an increased risk of developing several chronic illnesses, including dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, cardiovascular disease, and certain types of cancer. Obesity is a complex condition caused by a combination of genetic, environmental, and behavioral factors, including diet, physical activity, and exposure to endocrine-disrupting chemicals. It is characterized by an excess accumulation of body fat resulting from an ongoing positive energy balance (a higher intake of calories than expenditure) and insufficient physical activity, which disrupts the energy balance and normal physiological homeostasis. Growth hormone (GH), also referred to as the “master hormone”, exerts regulatory control over metabolic homeostasis and exerts multifaceted effects on numerous physiological processes.
1. The Negative and Positive Feedback Regulating Growth Hormone Production
The
growth hormone–insulin-like G
H–IGFrowth factor (GH–IGF) axis is a complex system that plays a crucial role in regulating growth and metabolism
[1,17,66][1][2][3]. The axis involves the interplay of several steroid hormones and proteins, which not only include GH and IGF-1 but also upstream signaling neuropeptides such as SST and GHRH, as well as the downstream signaling targets known as IGF-binding proteins (IGFBPs)
[65,79,80,81][4][5][6][7]. This section will focus on the mechanisms that regulate the negative and positive feedback effects in the GH–IGF axis.
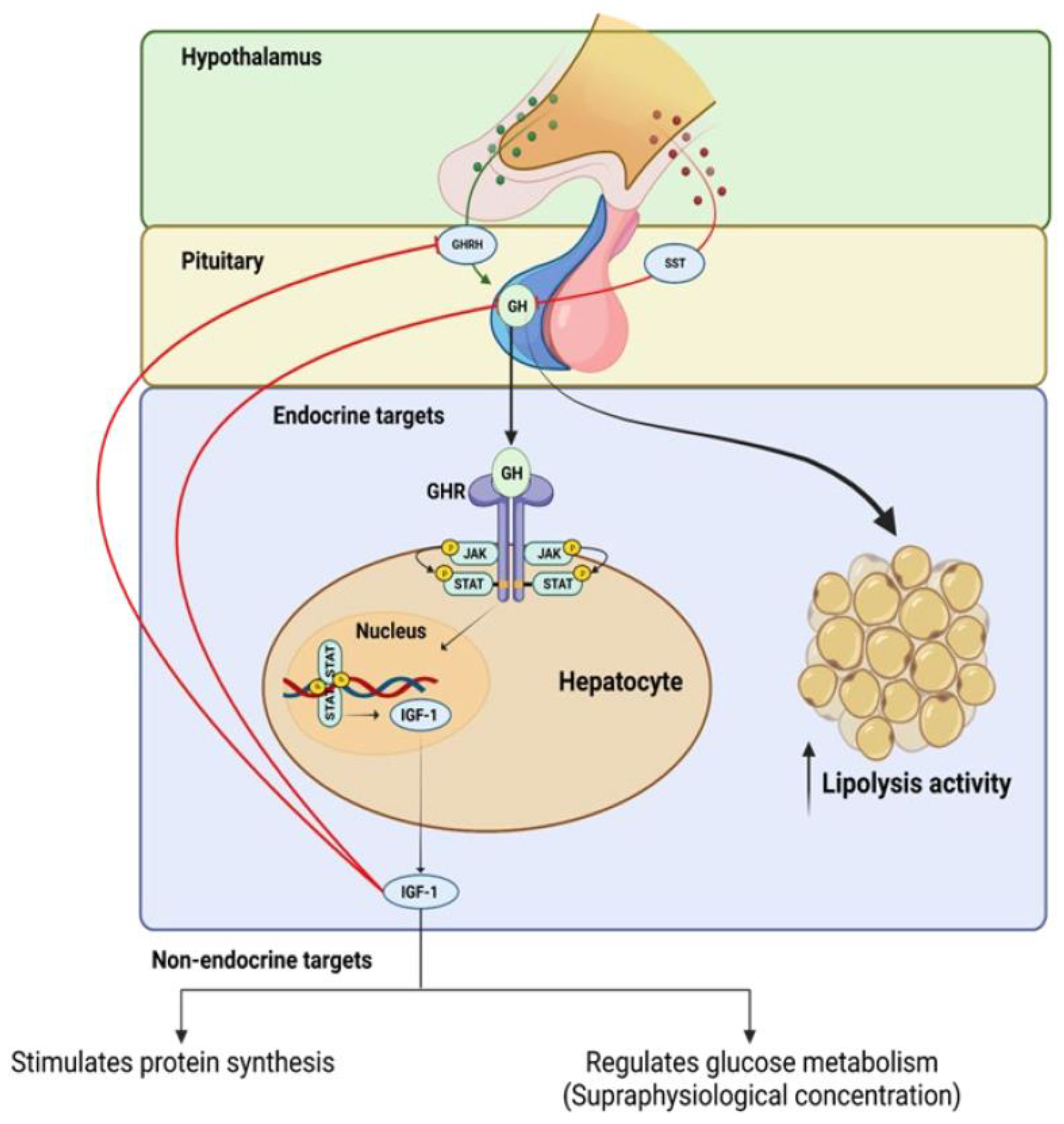
The GH–IGF axis operates through a positive and negative feedback loop, whereby GH, which is produced by somatotroph cells in the pituitary gland, stimulates the liver to produce circulating IGF-1 (see
Figure 1). This process is mediated by a pathway that leads to the activation of the transcription factor, signal transducer and activator of transcription 5 (STAT5) (see
Figure 1), which in turn stimulates the expression of IGF-1
[82][8]. The increased level of IGF-1 promotes cell growth and division by binding to its receptors on the cell surface and activating the PI3K/Akt signaling pathway
[5,83,84][9][10][11].
Figure 1.
Positive and negative feedback regulation of growth hormone (GH) production and intracellular mechanisms of growth hormone receptor (GhR) signaling.
One of the earliest studies that aimed to investigate the molecular mechanism by which IGF-1 suppresses GH gene expression at the pituitary level was conducted at Nagoya University in Japan. In that study, the researchers established a rat somatotroph tumor cell line, MtT/S, and transfected plasmids containing the GH 5′ promoter fused to the luciferase reporter gene. They found that IGF-1 suppressed GH promoter activity in a time and dose-dependent manner to inhibit GH secretion. Further experiments using deletion mutants of the GH promoter revealed that negative regulation was maintained on the shortest construct. This suggests that the IGF-1-related factor acts at the region nearest the minimal promoter. The study also found that the negative effect was eliminated by a PI3K inhibitor, indicating that the PI3K-mediated signaling pathway plays a major role in the negative regulation of IGF-1
[85][12].
A study was conducted to investigate how IGF-1 negatively regulates GH gene expression at the promoter level using a somatotroph cell line. The results revealed that IGF-1 inhibits GH mRNA levels by disrupting the binding of POU1F1 to the GH promoter through the inhibition of cyclic AMP (cAMP) response element binding protein (CBP). To confirm CBP’s role as a target of IGF-1R signaling, researchers used a mutant CBP construct and knock-in mouse model, which showed elevated serum GH levels, a greater response to GHRH stimulation, lower weight gain, and decreased body fat. The findings suggest that IGF-1R signaling disrupts the POU1F1/CBP complex to inhibit gene expression. Furthermore, chromatin immunoprecipitation assays demonstrated the inhibition of CBP binding to the GH promoter after IGF-1 treatment. Using a mutant CBP construct that lacked a critical phosphorylation site led to the loss of IGF-1 inhibition, supporting the hypothesis. The study confirmed the inhibitory effects of IGF-1 on GH expression at the promoter level and provided evidence of CBP’s role as a target of IGF-1R signaling
[86][13].
To further understand the role of IGF-1 in negative feedback,
ouresearcher
s' group developed a mouse model, referred to as the SIGFRKO mouse, in which the IGF-1R was ablated from the somatotroph and where they used the GH promoter as a driver for Cre recombinase. The SIGFRKO mice had increased GH gene expression and secretion and increased serum IGF-1. Additionally, the SIGFRKO mice had decreased GHRH and increased STT mRNA expression levels. These results support the idea that IGF-1 negatively regulates somatotroph synthesis and GH secretion, suggesting that hypothalamic feedback limits the extent of GH release
[18][14].
A recent study investigating the role of IGF-1 in regulating the negative feedback of GH secretion developed a different mouse model in which the IGF-1R was specifically ablated in GHRH-expressing cells
[84][11]. As expected, this mouse model presented an interesting phenotype that was characterized by an increase in GH levels, pulse amplitude, and frequency, as well as increased GHRH mRNA levels, GH mRNA expression, and serum IGF-1 levels, which were mediated by the pronounced elevation in the circulating level of GH. Despite the established role of GH in promoting lipolysis, this mouse model study did not demonstrate any effect on total fat mass despite the significant elevation in circulating GH levels
[18][14]. Recently,
ouresearcher
s' laboratory has developed a new mouse model, referred to as the S-GIGFRKO mouse, which is characterized by the ablation of the IGF-1R in both the somatotrophs and GHRH-expressing neurons. The results of
ourresearchers' study revealed that the S-GIGFRKO mouse line displayed a modest increase in circulating GH levels and circulating IGF-1 levels. Furthermore, the ablation of IGF-1R in this mouse model was associated with increased lipolysis activity and a decrease in total body fat mass. These findings demonstrate the crucial role of IGF-1 in regulating GH production through negative feedback mechanisms
[5][9]. Further details and analyses of these models will be provided in the next section of
ouresearcher
s' study.
In conclusion, the GH–IGF axis is a complex system that is tightly regulated to maintain the balance between growth and metabolic processes. SST, GHRH, and IGFBPs play important roles in regulating the positive and negative feedback in the axis, ensuring a homeostatic response to changing physiological conditions. These mechanisms are mediated by different signaling pathways and receptors that are activated by interacting hormones and proteins. Further research is required to fully understand the intricacies of this system and its potential therapeutic applications.
2. Overview of the Current Understanding of the Relationship between the Growth H–IGFormone–Insulin-Like Growth Factor-1 Axis and Obesity
GH and IGF-1 are endocrine signaling molecules that are critical for regulating processes such as growth, maturation, and metabolic homeostasis. It has been suggested that deviations from normal GH and IGF-1 levels are linked with the development of obesity; however, the precise mechanisms underlying this relationship have yet to be fully elucidated
[65,80,87][4][6][15].
One potential link between the GH–IGF-1 axis and obesity is the molecules’ effects on insulin sensitivity. IGF-1 has a nearly 50% amino acid sequence similarity with insulin and elicits an almost identical hypoglycemic response
[88,89][16][17]. The capability of IGF-1 to bind to insulin receptors suggests its involvement in mediating insulin activity. Several experimental models have been used to demonstrate the effect of IGF-1 on insulin sensitivity and resistance.
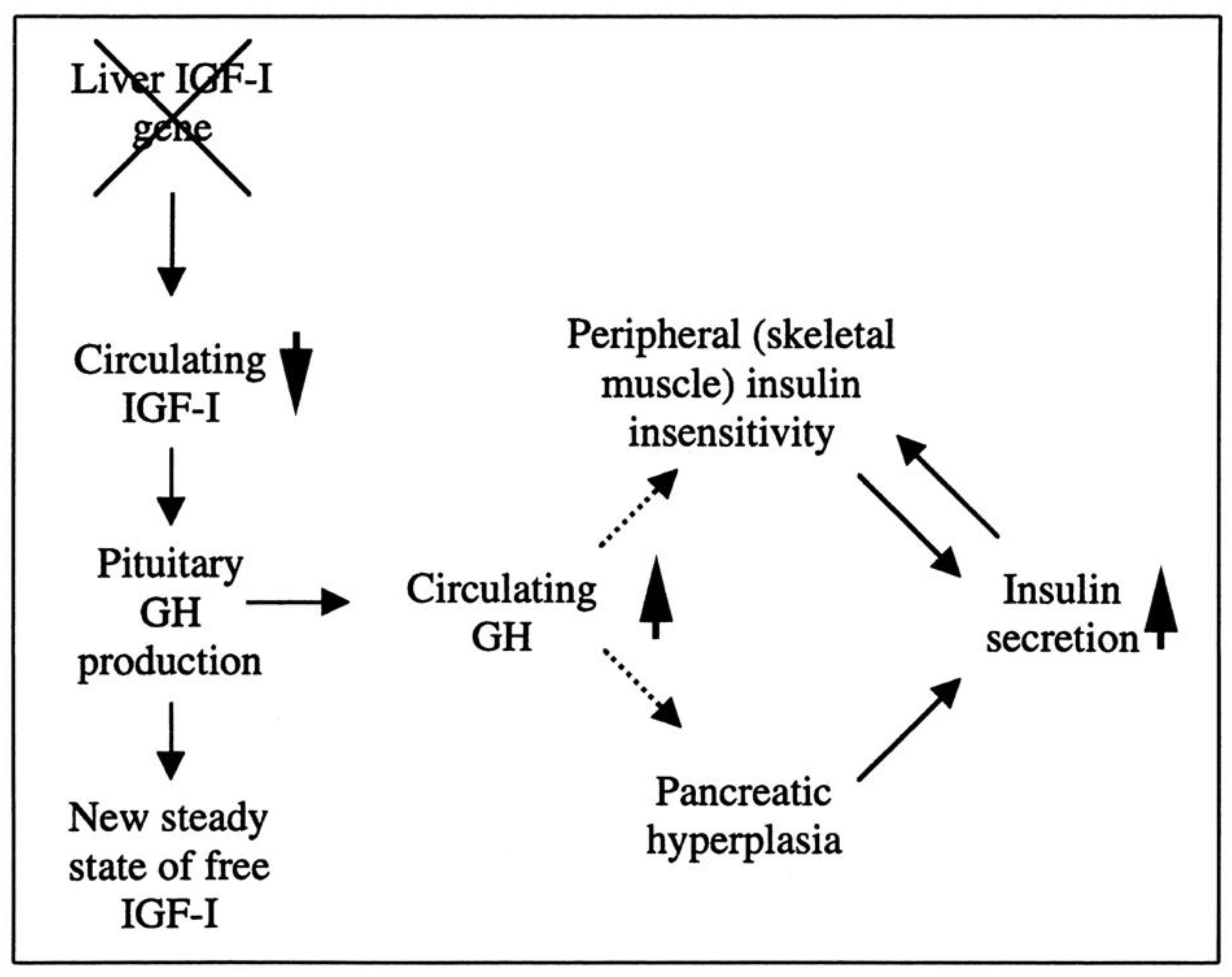
Researchers have created a mouse model known as the liver IGF-1-deficient (LID) mouse to investigate the metabolic effects of IGF-1 deficiency. The LID mice showed significantly reduced levels of IGF-1 and elevated levels of GH in their circulation, which is associated with higher insulin levels and abnormal glucose clearance after insulin injection. However, their fasting blood glucose levels and levels after a glucose tolerance test appeared to be normal. This suggests that the LID mice are insulin resistant but can maintain normal blood glucose levels due to the high insulin levels in their circulation. Treatment with recombinant human IGF-1 or a GH-releasing hormone antagonist, which reduces GH levels, improved insulin sensitivity in the LID mice. These findings indicate that circulating IGF-1 plays a role in insulin action in peripheral tissues (
Figure 2)
[90][18].
Figure 2. The role of liver-derived circulating IGF-I in muscle insulin sensitivity. Liver-specific igf-1 gene deletion results in reduced circulating total IGF-I and elevated GH levels, leading to insulin insensitivity in muscle and islet cell hyperplasia with hyperinsulinemia.
Evidence suggests that GH and IGF-1 could contribute to the onset of obesity by impacting inflammation and oxidative stress. A previous study aimed to investigate the potential role of GH and IGF-1 in the development of obesity and focused on their role in mediating oxidative stress and inflammation. The study determined the effects of long-term HFD-induced obesity on vascular function and metabolic alterations in a Lewis dwarf rat model of GH/IGF-1 deficiency. The results show that GH/IGF-1 deficiency exacerbates vascular dysfunction, inflammation, and oxidative stress when challenged with an HFD. However, GH/IGF-1 deficiency did not affect weight gain or changes in body composition in response to the HFD challenge. Instead, the low insulin levels observed in the GH/IGF-1 deficient rats may be due to compromised β-cell numbers or function and impaired β-cell compensation in response to metabolic challenges. GH/IGF-1 deficiency was associated with increased adiponectin levels but normal serum levels of leptin. In the control animals, the HFD stimulated an inflammatory response to increase circulating levels of multiple inflammatory cytokines, including IL-6 and TNF-α; however, the exact mechanism is not well understood
[91][19].
Another possible mechanism linking the GH–IGF-1 axis and obesity is through the effects on muscle mass and function and the development of adipose tissue. GH and IGF-1 are known to promote the growth and development of skeletal muscle, and low levels of these hormones have been associated with a reduction in muscle mass and function
[92][20]. This may lead to an impaired ability to burn calories, potentially contributing to weight gain. In addition, previous studies using several transgenic mouse models have demonstrated the crucial role of the GH–IGF-1 axis in regulating adipocyte proliferation and differentiation
[66,93][3][21]. Adipocyte differentiation is a complex process involving the activation of multiple transcription factors, signaling pathways, and epigenetic modifications
[94][22]. The process starts with the commitment of mesenchymal stem cells to the adipocyte lineage, followed by the formation of preadipocytes. Preadipocytes then undergo a series of morphological and biochemical changes to become mature adipocytes, which are characterized by the accumulation of triglycerides and the expression of adipocyte-specific genes, such as peroxisome proliferator-activated receptor gamma (PPARγ) and adiponectin
[95][23]. Finally, GH and IGF-1 may have effects on brain regions controlling the appetite
[81,96][7][24]. Dysregulation of GH and IGF-1 may therefore disrupt normal appetite control and contribute to weight gain.
The relationship between GH, IGF-1, and obesity is complex and not fully understood. Further research is needed to fully understand the mechanisms by which these hormones may be linked to the development of obesity and to identify potential strategies for preventing and treating obesity.
3. The Effect of Obesity on Growth GHHormone and IGFnsulin-Like Growth Factor-1 Production
Obesity is associated with a marked blunting of GH secretion, which is both spontaneous and is evoked by provocative stimuli. This reduction in GH secretion is observed in response to traditional pharmacological stimuli acting in the hypothalamus, such as insulin-induced hypoglycemia, arginine, galanin, L-dopa, clonidine, and acute glucocorticoid administration, as well as in response to direct somatotroph stimulation by exogenous GHRH
[115][25].
The impact of obesity on serum IGF-1 levels is a matter of controversy within the scientific community. While some studies have shown no alterations in IGF-1 levels in obesity, others have indicated a decrease in IGF-1 levels in the presence of obesity; others have also demonstrated an increase in IGF-1 levels in obese individuals
[65,116][4][26]. These seemingly contradictory findings may be explained by the high levels of insulin that are present in obesity, which has been shown to increase IGF-1 production in the liver while also reducing the formation of IGF-binding protein 1. This increased availability of free and active IGF-1, sustained by high insulin levels, may explain the decrease in GH secretion through negative feedback mechanisms. Therefore, understanding the mechanisms underlying the altered regulation of GH secretion in obesity is an important area of research as it may have implications for the treatment of obesity and related metabolic disorders.
Clinical trials have investigated the potential use of GH and IGF-1 as interventions for obesity. For example, A meta-analysis of 24 studies involving almost 500 obese individuals found that GH treatment led to a decrease in fat mass of about 1 kg and an increase in lean body mass of about 2 kg over an average of 12 weeks. The outcome of this
res
tudyearch suggests that treatment with recombinant human rhGH leads to a reduction in visceral fat and an increase in lean body mass in obese adults without causing weight loss. However, the treatment also leads to increases in fasting plasma glucose and insulin levels. The
studyresearch used relatively high doses of rhGH, and further studies with longer durations and lower doses are needed to better understand the effects of rhGH therapy on obesity and its potential impact on cardiovascular health
[117][27]. In all clinical studies that use rhGH as a therapeutic agent, caution is urged as its therapeutic safety is assessed. In summary, more research is needed to fully understand the effects of GH therapy and its potential side effects.