The fungal species belonging to the genus Trichoderma has been globally recognized as a potential candidate of biofertilizer and biocontrol agent to prevent devastating soil-borne fungal pathogens and enhance growth and productivity of agricultural crops. The antagonistic activity of Trichoderma to pathogenic fungi is attributed to several mechanisms including antibiosis and enzymatic hydrolysis, which are largely associated with a wide range of metabolites secreted by the Trichoderma species. Besides suppressing target pathogens, several metabolites produced by Trichoderma species may act against non-pathogenic beneficial soil microbial communities and perform unintended alterations within the structures and functions of microbial communities in the crop rhizosphere. Multiple microbial interactions have been shown to enhance biocontrol efficacy in many cases as compared to bioinoculant employed alone.
- Trichoderma
- metabolites
- root exudates
1. Trichoderma Species as a Commercial Biofungicide
| Trichoderma–Based Bioformulation/Tradename | Trichoderma Species | Target Pathogens | Manufacturer | References |
|---|---|---|---|---|
| Agroguard WG™ | T. harzianum | Phoma, Pythium, Rhizoctonia, Sclerotinia, and Sclerotium | Life Systems Technology S.A. (Colombia) | [34][8] |
| Antagon WP™ | T. harzianum | Botrytis, Ceratocystis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | Bio Ecologico Ltd. (Colombia) | [34][8] |
| Binab TF | T. polysporum | Fusarium, Pythium, Rhizoctonia, Sclerotinia, and Sclerotium | BINAB Bio-Innovation AB (USA) | [35][9] |
| Bioderma H | T. harzianum | Alternaria, Ascochyta, Cercospora, Colletotrichum, Fusarium, Phytophthora, Pythium, Macrophomina, Myrothecium, and Ralstonia | Biotech International Ltd. (India) | [34][8] |
| BioFungo™ | T. virens | Botrytis cinerea and Sphaerotheca pannosa | Orius Biotecnologia (Colombia) | [34][8] |
| ECO-77™ | T. harzianum | Botrytis and Eutypa | Plant Health Products (South Africa) | [34][8] |
| ECO-T™ | T. harzianum | Fusarium, Phytophthora, Pythium, and Rhizoctonia | Plant Health Products (South Africa) | [34][8] |
| Ecoderma | T. virens | Botrytis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | BigHaat Agro Ltd. (India) | [35][9] |
| Ecotrich ES™ | T. harzianum | Rhizoctonia solani, Pythium, and Sclerotinia | Ballagro Agro Tecnologia Ltd. (Brazil) | [34][8] |
| Esquive | T. atroviride | Pythium and Rhizoctonia | Agrauxine (France) | [35][9] |
| Floragard | T. hamatum | Fusarium, Pythium, Rhizoctonia solani, and Sclerotinia homeocarpa | Sellew Associates LLC (USA) | [35][9] |
| FoliGuard™ | T. hamatum | Alternaria, Botrytis cinerea, Cladosporium, Oidium, and Sphaeroteca pannosa | Live Systems Technology S.A. (Colombia) | [34][8] |
| Lycomax | T. viride | Soil-borne pathogens | Russell IPM (UK) | [34][8] |
| Natibiol™ | T. viride | Rhizoctonia | Probiagro S.A. (Venezuela) | [34][8] |
| PlantShield™/RootShield™ | T. harzianum | Fusarium, Pythium, Rhizoctonia solani, and Sclerotinia homeocarpa | Bioworks (USA) | [34][8] |
| T-Gro | T. harzianum | Botrytis, Ceratocystis, Fusarium, Pythium, and Rhizoctonia | Dagutat Biolab (USA) | [35][9] |
| Trianum™ | T. harzianum | Soil-borne pathogens | Koppert BV (The Netherlands) | [34,35][8][9] |
| Tricho™ | T. harzianum | Alternaria, Arrmilaria, Botrytis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | Orius Biotecnologia (Colombia) | [34][8] |
| Trichodermil™ | T. harzianum | Botrytis ricini, Fusarium, Phytophthora capsici, Phytophthora palmivora, Rhizoctonia, and Sclerotinia sclerotiorum | Itaforte BioProdutos (Brazil) | [34][8] |
| Trichodex | T. harzianum | Colletotrichum, Fusarium, Phytophthora, and Pythium, Macrophomina | Makhteshim chemical works Ltd. (USA) | [35][9] |
| Trichosav | T. harzianum | Soil-borne pathogens | Centros de Reproduccion de Medios Biologicos (Cuba) | [34][8] |
| Trichosoil | T. harzianum | Fusarium | Lage S.A. (Uruguay) | |
| Tusal | T. asperellum | Fusarium, Pythium, and Rhizoctonia solani | Isagro (USA) | [35][9] |
| Virisan | T. asperellum | Phytophthora, and Pythium | Isagro (USA) | [35][9] |
| VinevaxTM–Trichoprotection™ | T. harzianum | Armillaria, Botryosphaeria stevensii, Chondrostereum purpureum, Eutypa lata, and Phaeomoniella chlamydospor | Agrimm Technologies Ltd. (New Zealand) | [34,35][8][9] |
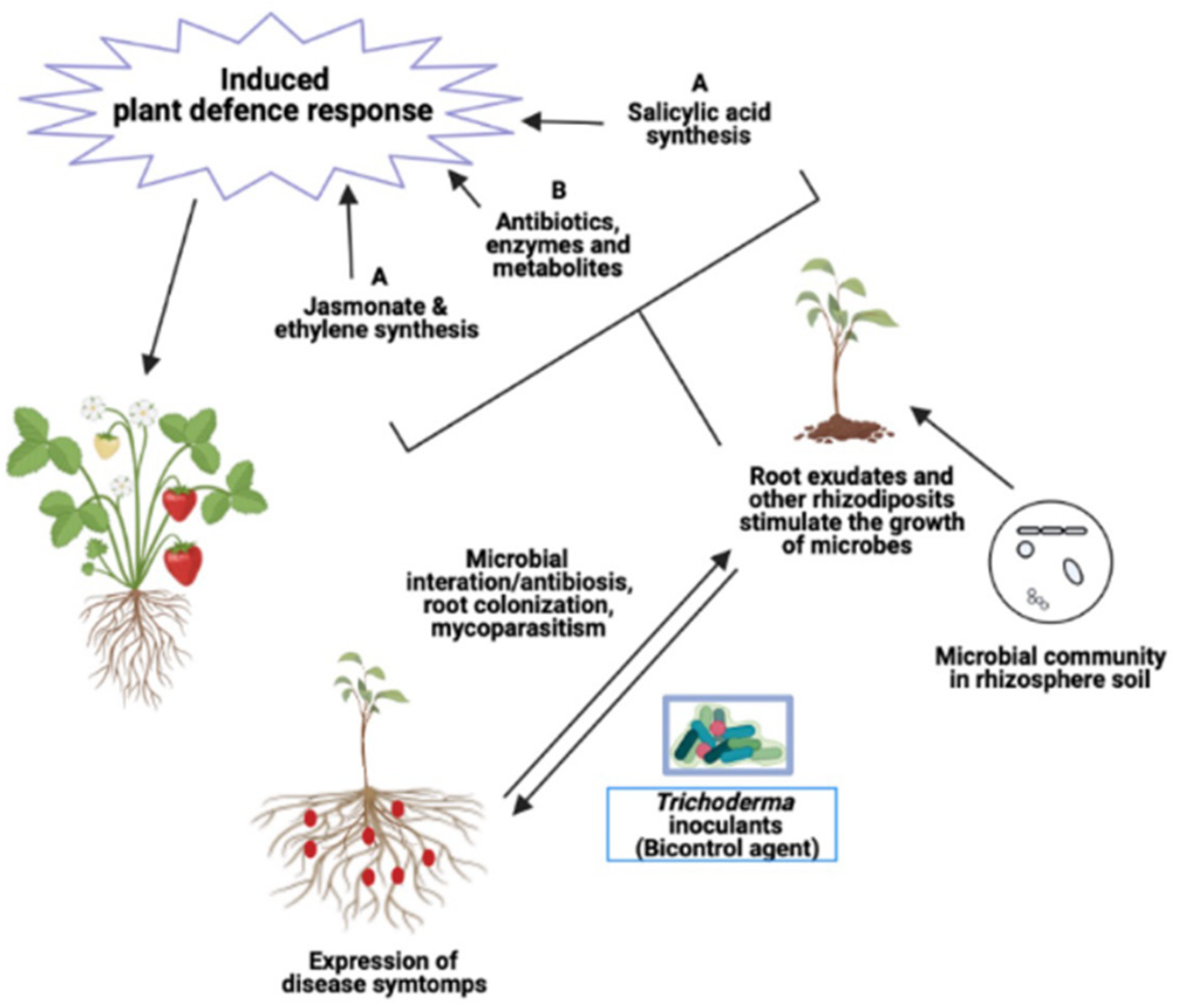
2. Effects of Trichoderma Metabolites on Plant Root Exudates
3. Effects of Trichoderma Metabolites on Soil and Root Pathogens
| Metabolites | Compound | Trichoderma Species | Target Fungal Pathogens | References |
|---|---|---|---|---|
| Anthraquinones | 1,8-dihydroxy-3-methylanthraquinone, 1-hydroxy-3-methylanthraquinone and 6-methyl-1,3,8-trihydroxyanthraquinone |
T. harzianum | Fusarium oxysporum, Macrophomina phaseolina, Rizoctonia solani, and Sclerotium rolfsii | [55][30] |
| 1,8-dihydroxy-3-methylanthraquinone and 1-hydroxy-3-methylanthraquinone |
T. harzianum | Gaeumannomyces graminis var. tritici, and Pythium ultimum | [56][31] | |
| Azaphilones | Harziphilone, Fleephilone and T22azaphilone |
T. harzianum | G. graminis var. tritici, P. ultimum, and R. solani | [57][32] |
| T22azaphilone | T. harzianum | Leptosphaeria maculans, and Phytophthora cinnamomi | [58][33] | |
| Epipolythiodio-xopiperazines | Gliotoxins | T. virens | M. phaseolina, Pythium aphanidermatum, Pythium deharyanum, Rizoctonia bataticola, R. solani, and S. rolfsii | [1][34] |
| Gliovirin | T. longibrachiatum | R. solani | [1][34] | |
| Gliovirin | T. virens | P. ultimum | [1][34] | |
| Koninginins | koninginins A, B, D, E, and G | T. aureoviride T. harzianum and T. koningii |
G. graminis var. tritici | [59][35] |
| koninginins A, B, and D | T. koningiopsis | F. oxysporum, F. solani, and S. rolfsii | [59][35] | |
| koninginin D | T. harzianum and T. koningii | Bipolaris sorokiniana, F. oxysporum, P. cinnamomi, and Pythium middletonii | [6][36] | |
| Lactones | aspinolide C | T. arundinaceum | Fusarium sporotrichioides | [60][37] |
| Cerinolactone | T. cerinum | Rosellinia necatrix | [60][37] | |
| Cremenolide | T. cremeum. | F. oxysporum and R. solani | [60][37] | |
| Peptaibols | trichokonins VI, VII, and VIII | T. koningii | F. oxysporum, R. solani, and Verticillium dahliae | [55][30] |
| Trichokonin VI | T. pseudokoningii | F. oxysporum, Phytophthora parasitica, and V. dahlia | [55][30] | |
| Polyketides | Harzianolide and Dehydro Harzianolide |
T. harzianum | F. oxysporum and R. solani | [61][38] |
| 6-pentyl-α-pyrone | T. harzianum T. koningii T. viride and Trichoderma spp. |
R. solani | [61][38] | |
| 6-pent-1-enyl-α-pyrone | T. harzianum and T. viride | R. solani | [61][38] | |
| Massoilactone δ-decenolactone | Trichoderma spp. | R. solani and S. rolfsii | [61][38] | |
| Koninginia E, B, and A | T. harzianum and T. koningii | G. graminis var. tritici | [57][32] | |
| Koninginin D and Seco-koninginin |
T. harzianum | G. graminis var. tritici | [57][32] | |
| Koninginin C | T. koningii | G. graminis var. tritici | [57][32] | |
| Pyridones | Harzianopyridone | T. harzianum | G. graminis var. tritici, L. maculans, P. cinnamomi, P. ultimum, and R. solani | [57][32] |
| Pyrones | 6-Pentyl pyrone (6-PP) | T. harzianum, T. koningii, and T. viride | F. oxysporum and R. solani | [62][39] |
| Viridepyronone | T. viride | S. rolfsii | [62][39] | |
| Steroids | Stigmasterol | T. harzianum and T. koningii | F. oxysporum, M. phaseolina, R. solani, and S. rolfsii, | [57][32] |
| Terpenes (trichothecenes) |
Trichodermin | T. polysporum T. sporulosum T. reesei and T. virens |
R. solani | [63][40] |
| Harzianum A | T. harzianum | F. oxysporum | [63][40] | |
| Mycotoxin T2 | T. lignorum | R. solani and S. rolfsii | [63][40] | |
| Terpenes (triterpenes) (sterols) |
Ergokonin A | T. koningii T. longibrachiatum and T. viride |
Phoma spp. | [64][41] |
| Viridin | T. koningii T. virens and T. viride |
F. oxysporum and R. solani | [64][41] | |
| Trichothecenes | Trichodermin | T. brevicompactum | R. solani | [65][42] |
| Trichodermin | T. harzianum | F. oxysporum and R. solani | [66][43] |
References
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329.
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133.
- Chaverri, P.; Samuels, G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 2013, 67, 2823–2837.
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590.
- Sudantha, I.M.; Sudirman; Ernawati, N.M.L. The effect of method and dosage application of biofungicide extract of Legundi leaf fermented with Trichoderma harzianum fungus for control of Fusarium wilt disease on shallots. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012014.
- Bailey, B.A.; Bae, H.; Strem, M.D.; Crozier, J.; Thomas, S.E.; Samuels, G.J.; Vinyard, B.T.; Holmes, K.A. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol. Control 2008, 46, 24–35.
- Adetunji, C.O.; Anani, O.A. Bio-fertilizer from Trichoderma: Boom for Agriculture Production and Management of Soil- and Root-Borne Plant Pathogens. In Innovations in Food Technology; Springer: Singapore, 2020; pp. 245–256.
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of Next-Gen agricultural challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063.
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126.
- Srivastava, M.; Kumar, V.; Shahid, M.; Pandey, S.; Singh, A. Trichoderma—A potential and effective biofungicide and alternative source against notable phytopathogens: A review. Afr. J. Agric. Res. 2016, 11, 310–316.
- Guerrieri, A.; Dong, L.; Bouwmeester, H.J. Role and exploitation of underground chemical signaling in plants. Pest Manag. Sci. 2019, 75, 2455–2463.
- Vargas, W.A.; Crutcher, F.K.; Kenerley, C.M. Functional characterization of a plant-like sucrose transporter from the beneficial fungus Trichoderma virens. Regulation of the symbiotic association with plants by sucrose metabolism inside the fungal cells. New Phytol. 2011, 189, 777–789.
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afric. J. Agric. Res. 2016, 11, 1952–1965.
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138.
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036.
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663.
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229.
- Chakraborty, B.N.; Chakraborty, U.; Sunar, K. Induced Immunity Developed by Trichoderma Species in Plants. In Trichoderma. Rhizosphere Biology; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 125–147.
- Srivastava, M.; Shahid, M.; Pandey, S.; Kumar, V.; Singh, A.; Trivedi, S.; Srivastava, Y.K.; Shivram. Trichoderma: A scientific approach against soil borne pathogens. Afr. J. Microbiol. Res. 2015, 9, 2377–2384.
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.-application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317.
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019, 20, 485.
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799.
- Harman, G.E.; Howell, C.R.; Viterbo, A. Trichoderma species opportunistic, a virulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56.
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353.
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019, 10, 1030.
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762.
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88.
- Esse, H.P.; Reuber, T.L.; Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86.
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi. 2021, 7, 61.
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817.
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma Secondary Metabolites Active on Plants and Fungal Pathogens. Open Mycol. J. 2014, 8, 127–139.
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Vinale, F.; et al. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303.
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal. Biol. Rev. 2014, 28, 97–125.
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control. 2018, 117, 147–157.
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal. Biol. Rev. 2016, 30, 74–90.
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma—A genomic perspective. Microbiology 2012, 158, 35–45.
- Vargas, W.A.; Mukherjee, P.K.; Laughlin, D.; Wiest, A.; Moran-Diez, M.E.; Kenerley, C.M. Role of gliotoxin in the symbiotic and pathogenic interactions of Trichoderma virens. Microbiology 2014, 160, 2319–2330.
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175.
- Degenkolb, T.; Fog Nielsen, K.; Dieckmann, R.; Branco-Rocha, F.; Chaverri, P.; Samuels, G.J.; Thrane, U.; von Döhren, H.; Vilcinskas, A.; Brückner, H. Peptaibol, Secondary-Metabolite, and Hydrophobin Pattern of Commercial Biocontrol Agents Formulated with Species of the Trichoderma harzianum Complex. Chem. Biodivers. 2015, 12, 662–684.
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–Strategies to activate silent gene clusters. Fungal. Genet. Biol. 2011, 48, 15–22.
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299.
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol Methods 2010, 81, 187–193.
- Rokas, A.; Wisecaver, J.H.; Lind, A.L. The birth, evolution and death of metabolic gene clusters in fungi. Nat. Rev. Microbiol. 2018, 16, 731–744.
