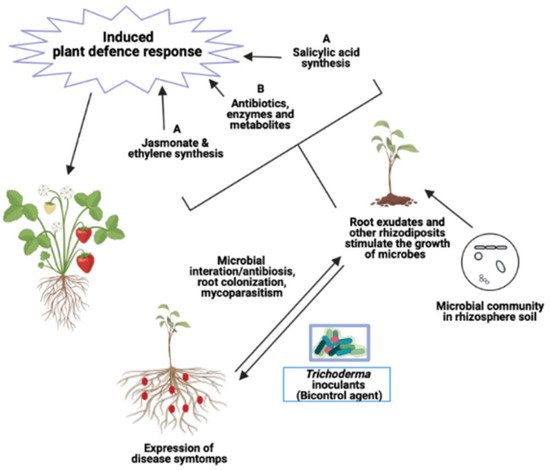
The fungal species belonging to the genus Trichoderma has been globally recognized as a potential candidate of biofertilizer and biocontrol agent to prevent devastating soil-borne fungal pathogens and enhance growth and productivity of agricultural crops. The antagonistic activity of Trichoderma to pathogenic fungi is attributed to several mechanisms including antibiosis and enzymatic hydrolysis, which are largely associated with a wide range of metabolites secreted by the Trichoderma species. Besides suppressing target pathogens, several metabolites produced by Trichoderma species may act against non-pathogenic beneficial soil microbial communities and perform unintended alterations within the structures and functions of microbial communities in the crop rhizosphere. Multiple microbial interactions have been shown to enhance biocontrol efficacy in many cases as compared to bioinoculant employed alone.
- Trichoderma
- metabolites
- root exudates
1. Trichoderma Species as a Commercial Biofungicide
| Trichoderma–Based Bioformulation/Tradename | Trichoderma Species | Target Pathogens | Manufacturer | References |
|---|---|---|---|---|
| Agroguard WG™ | T. harzianum | Phoma, Pythium, Rhizoctonia, Sclerotinia, and Sclerotium | Life Systems Technology S.A. (Colombia) | [8][34] |
| Antagon WP™ | T. harzianum | Botrytis, Ceratocystis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | Bio Ecologico Ltd. (Colombia) | [8][34] |
| Binab TF | T. polysporum | Fusarium, Pythium, Rhizoctonia, Sclerotinia, and Sclerotium | BINAB Bio-Innovation AB (USA) | [9][35] |
| Bioderma H | T. harzianum | Alternaria, Ascochyta, Cercospora, Colletotrichum, Fusarium, Phytophthora, Pythium, Macrophomina, Myrothecium, and Ralstonia | Biotech International Ltd. (India) | [8][34] |
| BioFungo™ | T. virens | Botrytis cinerea and Sphaerotheca pannosa | Orius Biotecnologia (Colombia) | [8][34] |
| ECO-77™ | T. harzianum | Botrytis and Eutypa | Plant Health Products (South Africa) | [8][34] |
| ECO-T™ | T. harzianum | Fusarium, Phytophthora, Pythium, and Rhizoctonia | Plant Health Products (South Africa) | [8][34] |
| Ecoderma | T. virens | Botrytis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | BigHaat Agro Ltd. (India) | [9][35] |
| Ecotrich ES™ | T. harzianum | Rhizoctonia solani, Pythium, and Sclerotinia | Ballagro Agro Tecnologia Ltd. (Brazil) | [8][34] |
| Esquive | T. atroviride | Pythium and Rhizoctonia | Agrauxine (France) | [9][35] |
| Floragard | T. hamatum | Fusarium, Pythium, Rhizoctonia solani, and Sclerotinia homeocarpa | Sellew Associates LLC (USA) | [9][35] |
| FoliGuard™ | T. hamatum | Alternaria, Botrytis cinerea, Cladosporium, Oidium, and Sphaeroteca pannosa | Live Systems Technology S.A. (Colombia) | [8][34] |
| Lycomax | T. viride | Soil-borne pathogens | Russell IPM (UK) | [8][34] |
| Natibiol™ | T. viride | Rhizoctonia | Probiagro S.A. (Venezuela) | [8][34] |
| PlantShield™/RootShield™ | T. harzianum | Fusarium, Pythium, Rhizoctonia solani, and Sclerotinia homeocarpa | Bioworks (USA) | [8][34] |
| T-Gro | T. harzianum | Botrytis, Ceratocystis, Fusarium, Pythium, and Rhizoctonia | Dagutat Biolab (USA) | [9][35] |
| Trianum™ | T. harzianum | Soil-borne pathogens | Koppert BV (The Netherlands) | [8][9][34,35] |
| Tricho™ | T. harzianum | Alternaria, Arrmilaria, Botrytis, Fusarium, Pythium, Rhizoctonia, Rosellinia, Sclerotinia, and Sclerotium | Orius Biotecnologia (Colombia) | [8][34] |
| Trichodermil™ | T. harzianum | Botrytis ricini, Fusarium, Phytophthora capsici, Phytophthora palmivora, Rhizoctonia, and Sclerotinia sclerotiorum | Itaforte BioProdutos (Brazil) | [8][34] |
| Trichodex | T. harzianum | Colletotrichum, Fusarium, Phytophthora, and Pythium, Macrophomina | Makhteshim chemical works Ltd. (USA) | [9][35] |
| Trichosav | T. harzianum | Soil-borne pathogens | Centros de Reproduccion de Medios Biologicos (Cuba) | [8][34] |
| Trichosoil | T. harzianum | Fusarium | Lage S.A. (Uruguay) | |
| Tusal | T. asperellum | Fusarium, Pythium, and Rhizoctonia solani | Isagro (USA) | [9][35] |
| Virisan | T. asperellum | Phytophthora, and Pythium | Isagro (USA) | [9][35] |
| VinevaxTM–Trichoprotection™ | T. harzianum | Armillaria, Botryosphaeria stevensii, Chondrostereum purpureum, Eutypa lata, and Phaeomoniella chlamydospor | Agrimm Technologies Ltd. (New Zealand) | [8][9][34,35] |
2. Effects of Trichoderma Metabolites on Plant Root Exudates
3. Effects of Trichoderma Metabolites on Soil and Root Pathogens
| Metabolites | Compound | Trichoderma Species | Target Fungal Pathogens | References |
|---|---|---|---|---|
| Anthraquinones | 1,8-dihydroxy-3-methylanthraquinone, 1-hydroxy-3-methylanthraquinone and 6-methyl-1,3,8-trihydroxyanthraquinone |
T. harzianum | Fusarium oxysporum, Macrophomina phaseolina, Rizoctonia solani, and Sclerotium rolfsii | [30][55] |
| 1,8-dihydroxy-3-methylanthraquinone and 1-hydroxy-3-methylanthraquinone |
T. harzianum | Gaeumannomyces graminis var. tritici, and Pythium ultimum | [31][56] | |
| Azaphilones | Harziphilone, Fleephilone and T22azaphilone |
T. harzianum | G. graminis var. tritici, P. ultimum, and R. solani | [32][57] |
| T22azaphilone | T. harzianum | Leptosphaeria maculans, and Phytophthora cinnamomi | [33][58] | |
| Epipolythiodio-xopiperazines | Gliotoxins | T. virens | M. phaseolina, Pythium aphanidermatum, Pythium deharyanum, Rizoctonia bataticola, R. solani, and S. rolfsii | [34][1] |
| Gliovirin | T. longibrachiatum | R. solani | [34][1] | |
| Gliovirin | T. virens | P. ultimum | [34][1] | |
| Koninginins | koninginins A, B, D, E, and G | T. aureoviride T. harzianum and T. koningii |
G. graminis var. tritici | [35][59] |
| koninginins A, B, and D | T. koningiopsis | F. oxysporum, F. solani, and S. rolfsii | [35][59] | |
| koninginin D | T. harzianum and T. koningii | Bipolaris sorokiniana, F. oxysporum, P. cinnamomi, and Pythium middletonii | [36][6] | |
| Lactones | aspinolide C | T. arundinaceum | Fusarium sporotrichioides | [37][60] |
| Cerinolactone | T. cerinum | Rosellinia necatrix | [37][60] | |
| Cremenolide | T. cremeum. | F. oxysporum and R. solani | [37][60] | |
| Peptaibols | trichokonins VI, VII, and VIII | T. koningii | F. oxysporum, R. solani, and Verticillium dahliae | [30][55] |
| Trichokonin VI | T. pseudokoningii | F. oxysporum, Phytophthora parasitica, and V. dahlia | [30][55] | |
| Polyketides | Harzianolide and Dehydro Harzianolide |
T. harzianum | F. oxysporum and R. solani | [38][61] |
| 6-pentyl-α-pyrone | T. harzianum T. koningii T. viride and Trichoderma spp. |
R. solani | [38][61] | |
| 6-pent-1-enyl-α-pyrone | T. harzianum and T. viride | R. solani | [38][61] | |
| Massoilactone δ-decenolactone | Trichoderma spp. | R. solani and S. rolfsii | [38][61] | |
| Koninginia E, B, and A | T. harzianum and T. koningii | G. graminis var. tritici | [32][57] | |
| Koninginin D and Seco-koninginin |
T. harzianum | G. graminis var. tritici | [32][57] | |
| Koninginin C | T. koningii | G. graminis var. tritici | [32][57] | |
| Pyridones | Harzianopyridone | T. harzianum | G. graminis var. tritici, L. maculans, P. cinnamomi, P. ultimum, and R. solani | [32][57] |
| Pyrones | 6-Pentyl pyrone (6-PP) | T. harzianum, T. koningii, and T. viride | F. oxysporum and R. solani | [39][62] |
| Viridepyronone | T. viride | S. rolfsii | [39][62] | |
| Steroids | Stigmasterol | T. harzianum and T. koningii | F. oxysporum, M. phaseolina, R. solani, and S. rolfsii, | [32][57] |
| Terpenes (trichothecenes) |
Trichodermin | T. polysporum T. sporulosum T. reesei and T. virens |
R. solani | [40][63] |
| Harzianum A | T. harzianum | F. oxysporum | [40][63] | |
| Mycotoxin T2 | T. lignorum | R. solani and S. rolfsii | [40][63] | |
| Terpenes (triterpenes) (sterols) |
Ergokonin A | T. koningii T. longibrachiatum and T. viride |
Phoma spp. | [41][64] |
| Viridin | T. koningii T. virens and T. viride |
F. oxysporum and R. solani | [41][64] | |
| Trichothecenes | Trichodermin | T. brevicompactum | R. solani | [42][65] |
| Trichodermin | T. harzianum | F. oxysporum and R. solani | [43][66] |