Alzheimer’s Disease (AD), the most common type of dementia, is known as a neurodegenerative disease caused by the accumulation of amyloid beta (Aβ) peptides and tau protein hyperphosphorylation resulting in the formation of neurofibrillary tangles, activation of inflammasomes, sluggish autophagy, and neuronal loss. Several of these hallmarks are linked to alteration in the gut microbiome, also known as gut dysbiosis. Selective bioflavonoids can target gut microbiome to inhibit inflammasomes and resume autophagy to stop AD pathogenesis. Two bioflavonoids, specifically epigallocatechin-3-gallate (EGCG) and genistein (GS), appear to be a new paradigm of treatment for maintaining healthy gut microbiome in AD via modulating crucial AD signaling pathways.
- Alzheimer’s disease (AD)
- autophagy
- bioflavonoids
- epigallocatechin-3-gallate (EGCG)
- genistein (GS)
- gut microbiome
1. Introduction
2. Bioflavonoids as Novel Therapeutic Option for AD
There are varying potential categories for therapeutic values of acetylcholinesterase enzyme (AChE) inhibitors in the treatment of AD. Polyphenols are known to have enormous potential to regulate diversity as well as the composition of gut microbiota, which is associated with neurological health. Studies have showcased that polyphenols can reduce the neurological deficits that are caused due to neuroinflammation [10][11][12]. Bioflavonoids are a group of natural polyphenolic compounds that are derived from fruits and vegetables. A few examples of fruits and vegetables would include apples, onions, mulberries, and bilberries. Bioflavonoids are popularly consumed via tea, beer, and wine [13]. This subclass of polyphenols of biological origin is implicated in anti-apoptotic and pro-survival signaling pathways and decreasing the pathological effects of AD [14][15]. Bioflavonoids, which are exclusively derived from biological origins (mainly plants), are also well known for showcasing anti-inflammatory, anti-viral, anti-apoptotic, anti-platelet, and anti-tumoral properties [13][18][19]. Catechins are a group of bioflavonoids that can be extracted from tea, and this group includes epigallocatechin (EGC), epicatechin gallate (ECG), epicatechin (EC), and the most abundant compound EGCG [19]. As shown in Figure 1, the chemical structure of EGCG contains A, B, C, and D rings produced from the esterification of EGC with gallic acid [20]. Both the A and C rings have a phenyl group at C2 and a gallate group at C3 positions. The B and D rings of EGCG contain 3,4,5-trihydroxy groups, which have the potential for proteasome activity in vitro [20]. On the aromatic B ring, catechins have di- or tri-hydroxyl groups along with meta-5,7-dihydroxyl groups on the A ring [21]. The presence of phenolic groups in these compounds increases their antioxidant properties. The structure of flavonoids is important in creating a novel therapeutic drug for the treatment of AD.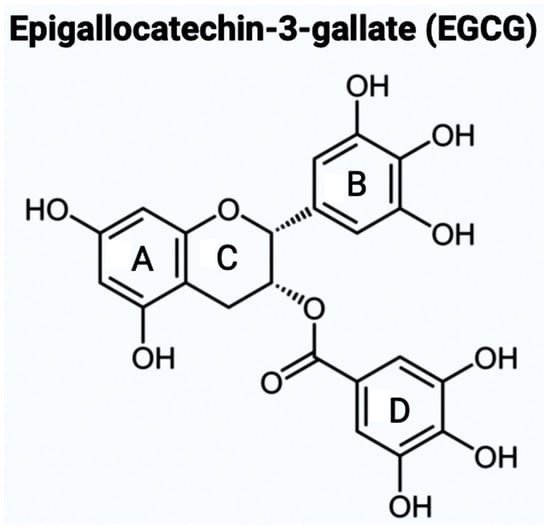
3. An Overview of the Gut Microbiota
The gut microbiota in every individual is unique, and thus, it is determined through environmental factors rather than being a genetically inheritable trait. In the gut microbiota, the staggering microbial diversity and colonization result in varying complex interactions, diseases, and immune responses. Depending on the area of the gastrointestinal tract being examined, the density and diversity of the gut bacteria fluctuate due to the difference in the local conditions [25]. A proper understanding of the function of microbiota in the gut is crucial for the development of successful therapeutics to target neurological diseases including AD.3.1. Activity of Gut Microbiota in the Human Body
The intestinal bacteria produce short-chain fatty acids (SCFAs) through the fermentation of non-digestible carbohydrates (NDC) and dietary fiber, and these SCFAs are formate, acetate, propionate, and butyrate, with the presence of acetate being three times higher [26][27][28]. Specifically, an increased presence of formate has been linked to the possibility of higher inflammation [28]. In a study of the introduction of butyrate to isolated germ-free colonocytes, the rate of oxidative phosphorylation increased while autophagy decreased [27]. For fermentation, the source of the carbohydrates used is the ones that were not digested or absorbed in the small intestine [28]. The functions of SCFAs include affecting cellular processes such as gene expression, differentiation, proliferation, and apoptosis [27]. The SCFAs, which are produced from NDC and dietary fiber, also regulate the permeability of the gut and blood-brain barriers [29]. In the CNS, SCFAs play a key role during the production of neural progenitor cells (NPCs) that produce neuronal and glial cell types [30][31]. According to a study conducted recently, the increased concentration of SCFAs positively affected the expression of genes involved in the proliferation of NPCs [32]. Free fatty acids (FFAs), which are the products of the metabolic pathways, are known to function as signaling molecules via interaction with free fatty acid receptors (FFARs) that form a family of G protein-coupled receptors (GPCRs). As the largest group of transmembrane proteins, GPCRs are currently known to be the most successful drug targets. There are a few mechanisms that are regulated by SCFAs to increase the production of NPCs. The first mechanism involves specific FFARs (i.e., GPCRs), most notably FFAR2 (GPR43) and FFAR3 (GPR41), being upregulated due to increased exposure to SCFAs. In the second mechanism, SCFAs regulate the physiological pH that modulates neurodevelopmental effects along with anti-apoptotic effects [32].3.2. Onset Factors in the Microbiota for Dysbiosis
Dysbiosis occurs when the normal state of the gut microbiota is unbalanced due to varying factors. One of the main examples is when the anti-inflammatory cytokines and the pro-inflammatory cytokines produced by the microbes are not balanced, then dysbiosis takes place. There are three types of dysbiosis: type 1 indicates a decrease of beneficial bacteria, type 2 shows an increase of pathogenic bacteria, and type 3 states a decrease in overall bacterial diversity [34]. The number of factors that can directly affect dysbiosis are many such as diet, birthing conditions (e.g., mode of birth, antibiotic exposure, and hygiene), chemical exposure, psychological and environmental stimuli (e.g., pathogens, sleep deprivation, circadian rhythm dysfunction, toxins, and noise), temperature, and intestinal infection. Diet is one of the crucial regulators of the gut microbiota [26]. In studies comparing a ‘Western diet’ (high animal protein, high in sugar and saturated fats) and an ‘agrarian diet’ (low animal protein, low levels of saturated fat and simple sugars), the results displayed the ‘Western diet’ leading to dysbiosis and lower levels of SCFAs [35]. On the other hand, the ‘agrarian diet’ results in more production of SCFAs and higher gut bacteria diversity, which helps limit the growth of potentially pathogenic bacteria that otherwise lead to diseases such as IBD [35]. The reason why the lower animal protein levels in an ‘agrarian diet’ help with the gut microbiota is due to the side effects of protein and amino acid fermentation [36]. When more protein is consumed, the gut must shift to increase the pH to break down the proteins that result in the production of compounds, including hydrogen sulfide, reactive oxygen species, and ammonia, which are unhealthy for the gut [36][37].3.3. Gut-Brain Axis (GBA) and Gut Dysbiosis
The relationship between the gut microbiota and the brain is called the gut-brain axis (GBA), and this two-way communication is built using immune, circulatory, and neural pathways (Figure 3) [30]. GBA connects the CNS (comprised of brain and spinal cord), autonomic nervous system (ANS), enteric nervous system (ENS), and hypothalamic pituitary adrenal (HPA) axis [38]. Particularly, the function of the HPA axis includes regulating the adaptive responses from the body to any stressors needed [38][39]. An increase in the occurrence of inflammatory cytokines such as interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) through the production of corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH) is an example of environmental stress that can activate the HPA axis. The bidirectional communication line results in the regulation of intestinal functional effector cells (immune cells, epithelial cells, enteric neurons, smooth muscle cells, interstitial cells of Cajal, and enterochromaffin cells) [38]. Unsurprisingly, the gut microbiota has been implicated in affecting the bidirectional communication between the gut and the brain [38]. The microbes present in the gut produce metabolites such as SCFAs, gamma-aminobutyric acid (GABA), tryptophan, serotonin, catecholamines, metabolites of bile acids and neurotransmitters, and cytokines that can signal to the receptors present in the gut [40]. The dysbiosis of the gut microflora causes an increase in the gut and blood-brain barrier permeability, production of bacterial amyloids, and formation of lipopolysaccharides (LPS) leading up to the deposition of amyloid fibrils in the brain, resulting in the pathogenesis (neuroinflammation, cognitive decline) of neurological disorders such as AD and stroke [41]. There still has not been a complete understanding of the pathways involved in the GBA bidirectional communication line.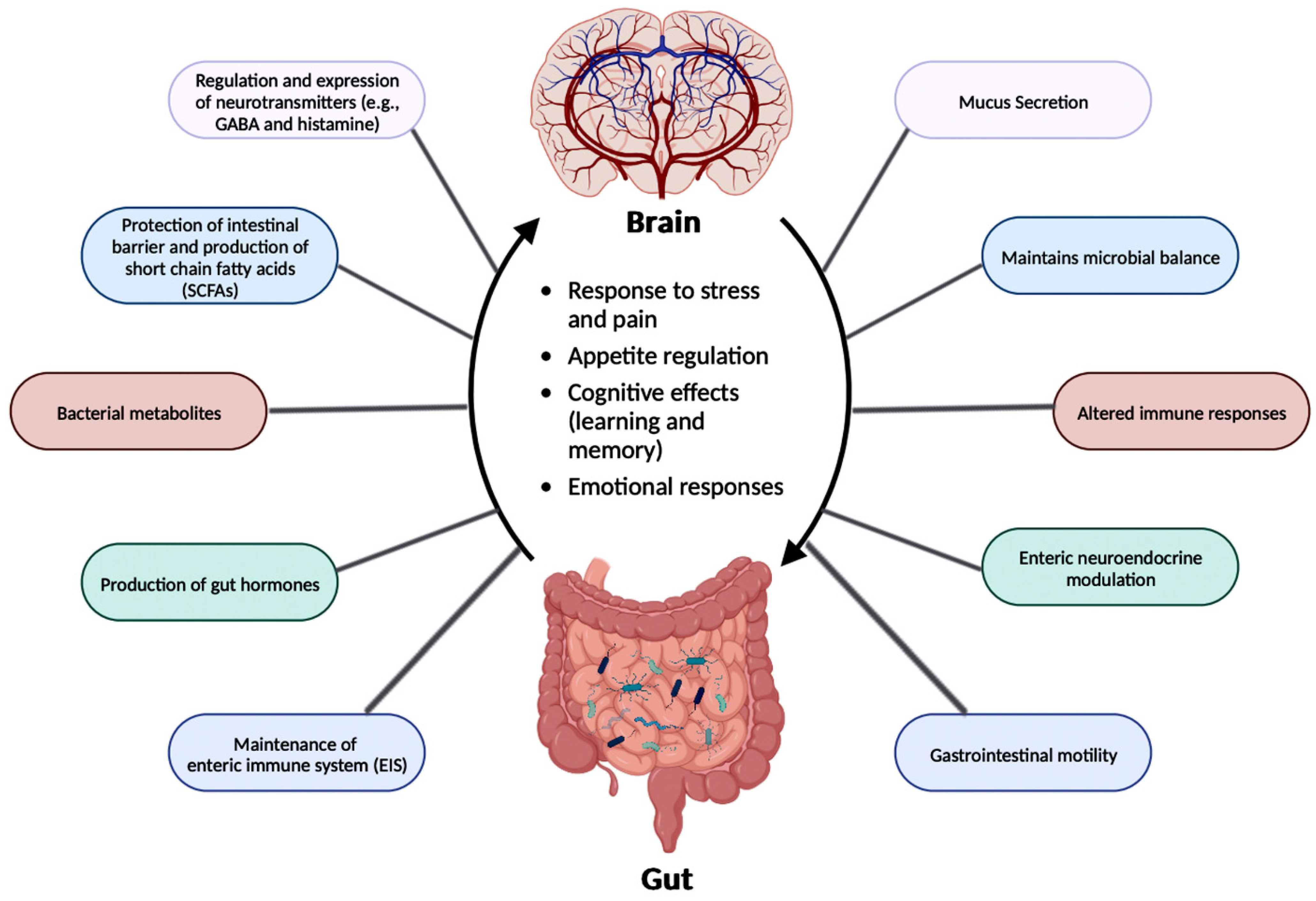
3.4. GBA and AD
The dysbiosis in gut microbiota directly affects the GBA, which is linked to AD clinical symptoms such as Aβ plaque deposition, cognitive decline, and memory loss (Table 1). To explore the change in the microbiota and the Aβ plaque deposition in the brain, studies created AD animal models comparing the microorganisms and SCFAs in fecal samples between AD mice and wild-type animals [42]. The AD mice displayed lower levels of SCFAs, which had the potential to alter multiple metabolic pathways along with increasing the deposition of Aβ plaques [42]. Another study for analyzing the effect of age compared the double transgenic (TG) mice expressing a chimeric mouse/human amyloid precursor protein (APP) and a mutant human presenilin 1 (PSEN1), both APP/PSEN1 mutations ensured an early-onset of D, and C57BL/6 wild-type (WT) mice, and the results unveiled the potential of targeting the gut microbiota in AD animals [43]. The 6-month-old APP/PSEN1 mice, with their gut microflora documented differently from the WT mice, experienced cognitive decline [43]. The microbial diversity of the APP/PSEN1 mice deteriorated along with age with increases in the population of bacteria from the Helicobacteraceae and Desulfovibrionaceae families [43]. In the Helicobacteraceae family, Helicobacter pylori (H. pylori) participates in causing dysbiosis resulting in gastric disorders such as chronic active gastritis, peptic ulcer disease (PUD), mucosa-associated lymphoid tissue (MALT) lymphoma and gastric carcinoma [44]. The decrease in microbial diversity in the APP/PSEN1 mice highlights the importance of regulating the gut microbiome as a viable therapeutic target for the treatment of AD.| AD Animal Model | Change in Gut Microbiota in AD Mice |
Observed Pathological Symptoms |
Reference |
|---|---|---|---|
| AD model mice (with varying ages) | Decreased microbial diversity and reduced SCFA levels | Amyloid deposition and ultrastructural abnormalities in the intestine, cognitive dysfunction, and signaling pathway alterations | [42] |
| APP/PSEN1 mice | Decreased microbial diversity | Cognitive dysfunction | [43] |
| APPSWE/PSIΔE9 mice | Varied gut microbial composition | Increased cerebral Aβ pathology | [46] |
| APP/PS1 mice | Increased pro-inflammatory bacteria during aging | Autism and inflammatory-related disorders | [47] |
| ApoE-/- mice | Porphyromonas gingivalis infection | Neuronal injury | [48] |
4. Neuroinflammation in AD
Most neurological conditions, such as autism spectrum disorders (ASD), epilepsy, PD, cerebrovascular diseases, and AD, have aspects of neuroinflammation as part of their pathogenesis. Cytokines, produced by microglia and astrocytes, are the central factors that influence all characteristics of neuroinflammation, ranging from pro-inflammatory and anti-inflammatory processes to neuronal injury [50].4.1. Implications of Gut Microbiota in Neuroinflammation in AD
A trigger for inflammation is the structural components of bacteria, including the by-products (e.g., SCFAs, enzymes, metabolites, LPS, cell capsule carbohydrates, and endotoxins) produced during the metabolic processes involved [51]. Chronic low-grade inflammation or inflammaging is found to cause tissue damage in most age-related diseases, including AD. One of the causes of inflammaging is dysbiosis in the gut microbiota [52]. Studies exploring the inflammation caused by an imbalance in intestinal immunity confirm the involvement of gut microbiota in innate and adaptive immunity, especially in IBD, which eventually can result in PD [28]. These studies use sterile-raised germ-free (GF) mice lacking the microorganisms existing in the gastrointestinal (GI) tract, along with mice without pathogens treated with broad-spectrum antibiotics (ABX). The ABX mice represented the innate immune system in which the myeloid cells in the bone marrow were impaired, resulting in a decrease of granulocytes and, thus, a higher likelihood of bacterial infection. In the GF mice, the development of innate lymphoid cells (ILCs) was disabled, leading to antigen receptors not being expressed. Additionally, this lack of expression affects enteric bacterial infections because the production of IL-22 decreases [28]. Considering the pathogenesis of PD is closely related to AD, the triggers for extreme inflammation could be shared between the two proteinopathies (aberrant protein aggregate diseases). The prominent inflammatory markers generated by the gut microbiota include LPS, SCFAs, bile acids (BAs), C-reactive protein (CRP), and cytokines. The first marker mentioned, LPS, also called endotoxin, is a part of the cell wall of Gram-negative bacteria [54]. In a normal gut state, LPS (concentration ranges from 0 to 1.0 ng·mL−1) is prevented by the gut barrier (intestinal epithelial and mucosal layers) from entering systemic circulation and activating epithelial destruction [55][54]. However, as shown in Figure 4, LPS increased enterocyte membrane TLR-4 expression in animal models of inflammation [55].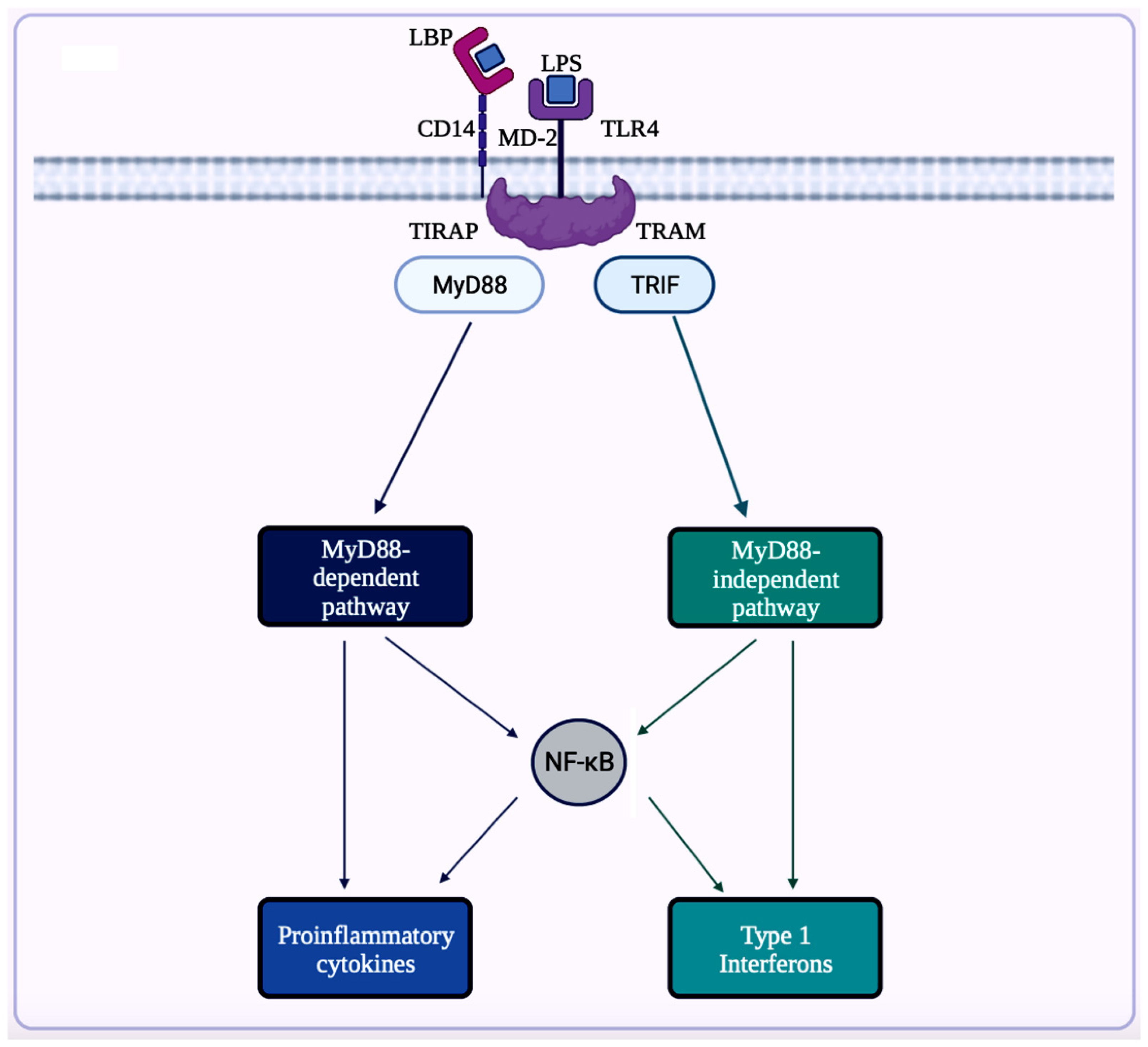
4.2. Inflammasomes in AD
Inflammasome, which is a cytosolic multiprotein complex, is implicated in causing excessive inflammation in various diseases, including autoimmune diseases, cancers, and neurodegenerative diseases [59]. In the innate immune system, if adverse stimuli (e.g., pathogens, dead cells) are detected, then inflammasomes are the receptors deployed to activate caspase-1, which contains a caspase recruitment domain (CARD), resulting in inflammation [51]. The innate immune response cascade begins with the initiation of germline-encoded pattern recognition receptors (PRRs), transmembrane or cytosolic receptors, by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [60]. PAMPs are the structural moieties present in microorganisms such as Gram-negative bacterial LPS, bacterial or viral nucleic acids, and bacterial peptides (e.g., flagellin). DAMPs are the endogenous molecules activated due to cellular stress, such as chromatin-associated proteins, heat-shock proteins, uric acid, and extracellular matrix fragments [60]. After the PAMPs or the DAMPs activate the cascade, PRRs such as nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), absent in melanoma-2 (AIM-2)-like receptors (ALRs), and tripartite motif-containing (TRIM) proteins form inflammasomes. There are three diverse types of inflammasomes: NLR-associated inflammasomes, ALR-associated inflammasomes, and the pyrin inflammasome [59]. Inflammasomes can be activated through two kinds of inflammasome signaling such as canonical and non-canonical [54]. The canonical inflammasome signaling consists of one or more inflammasome sensors, such as apoptosis-associated speck-like protein containing CARD (ASC) and caspase-1. ASC has a bipartite structure with a pyrin domain (PYD) and a CARD, both of which aid ASC in acting as an adaptor molecule. The non-canonical signaling pathway includes the activation of mouse caspase-11 or human caspase-4 and caspase-5 [51][60]. The NLRP3 (NOD-, leucine-rich repeat- or LRR-, and PYD-containing protein 3) inflammasome is a multimeric protein complex – which is made up of the sensor protein NLRP3, the adaptor protein ASC, and the effector protein pro-caspase-1 – having a role in development of AD [61][62][63]. When the sensor protein NLRP3 is activated, it binds to the PYD of the adaptor protein ASC, resulting in the cleavage of pro-caspase-1 into activate caspase-1 to form the NLRP3 inflammasome (Figure 5) [64]. This activates caspase-1, which then activates the inactive pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into their respective mature forms [64]. Along with activating cytokines, the activated caspase-1 can also cause pyroptosis, an inflammatory-related programmed cell death [64]. Among the family of inflammasomes, NLRP3 is the most extensively studied [63].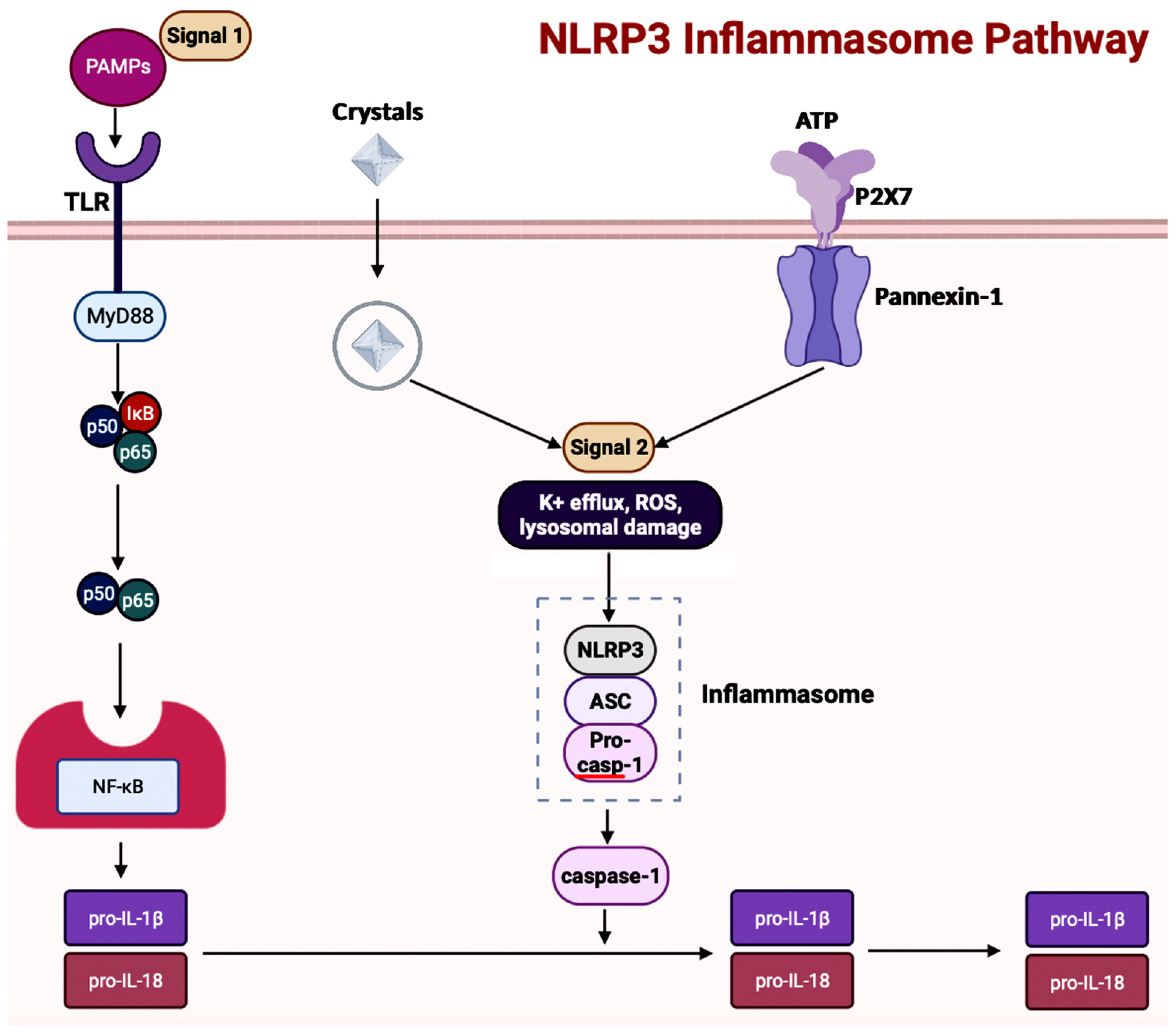
4.3. Inflammasomes and GBA in AD
Inflammasome activity is influenced by alterations in the gut microbiota and diet [58]. In a ketogenic diet or calorie restriction, the NLRP3 inflammasome gets inhibited because the ketone body β-hydroxybutyrate production in the liver increases [62]. A study exploring sickness-induced anorexia analyzed the relationship between Salmonella typhimurium and the GBA [69]. The S. typhimurium effector, Slrp, was used to inhibit the inflammasome pathway to hinder anorexia [69]. In the blood and brain samples collected from patients experiencing cognitive decline, an overexpression of NLRP3 in astrocytes and microglia resulting in central inflammation was witnessed [70]. Contrastingly, the same study also analyzed the effects of dysbiosis on the activation of peripheral inflammation, consisting of the innate cells in the gastrointestinal (GI) tract. The results showed that activation of peripheral inflammasomes triggered NLRP3-mediated neuroinflammation in the brain [70]. In a study conducted, the gut microbiota from AD patients was transferred to APP/PSEN1 mice, causing microglial and NLRP3 inflammation leading to the release of inflammatory factors [71]. The GI tract then absorbs the inflammatory factors to cause inflammation. So, targeting the inflammasome signaling pathway through improving the composition of the gut microbiota would be a possible therapeutic option in AD.5. Autophagy in AD
In AD brains, the main pathological changes are the deposition of Aβ plaques and intracellular NFTs made from hyperphosphorylated tau proteins [72][73]. The accumulation of Aβ plaques begins from the dysregulation of Aβ, which is modulated by autophagy [73]. Figure 6 showcases autophagy, which is a self-degradative process that plays a critical role in maintaining cellular homeostasis [74][75]. Autophagy is required to degrade the misfolded proteins present in the brain to prevent neurodegenerative diseases. Further studies have shown that the expression of autophagy-related proteins is downregulated in AD, implying the importance of autophagy upregulation as a part of treatments for AD [76].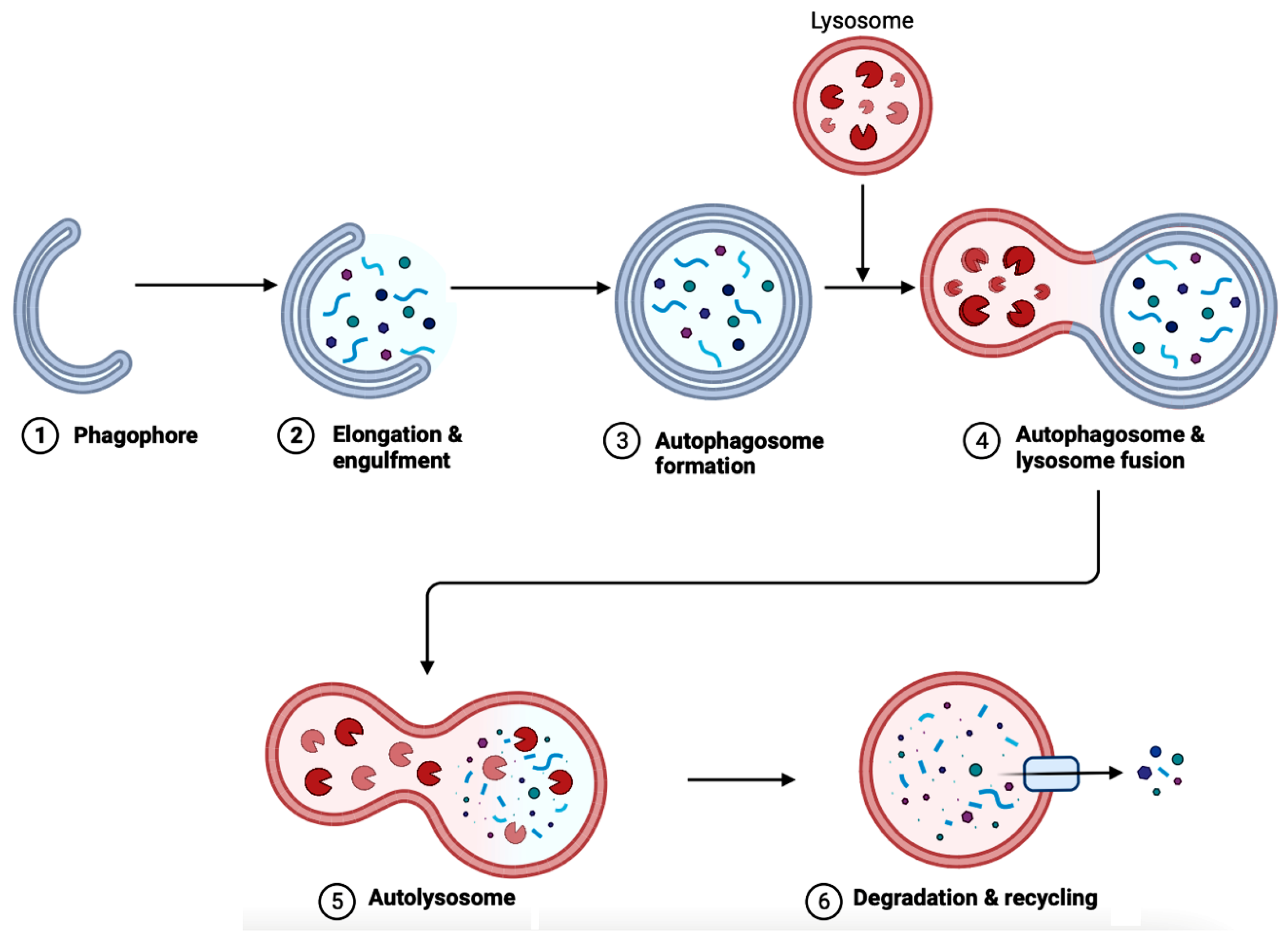
5.1. Implications of Gut Microbiota in Autophagy in AD
The gut microbiota has direct implications on many factors, including autophagy due to the existence of GBA. One of the ways to scrutinize whether gut flora has any effect on autophagy is to use GF mice. In the colonic epithelium of GF mice, the level of basal autophagy decreased compared to mice with intact gut flora [80]. The study also reinstated intestinal autophagy in vivo using butyrate-producing bacterial stain Butyrivibrio fibrisolvens. Bacteria-derived metabolites other than butyrate, such as indole-3-lactate produced by Lacticaseibacillus, Lactobacillus, Bifidobacterium, Megamonas, Roseburia, or Ruminococcus are also alternative options to induce intestinal autophagy [80]. These bacteria-derived metabolites can also modulate intestinal inflammation through the autophagy pathway. E. coli regulates autophagy through the NF-κB pathway, which upregulates selective microRNAs (miRNAs) before inhibiting ATG-specific proteins and then autophagy [81]. Dysfunctional autophagy and gut dysbiosis are both in a positive feedback loop due to dysregulated autophagy resulting in impaired intestinal epithelial barrier function through altering the levels of expression of the CLDN2 (Claudin-2) gene that codes for the tight junction protein Claudin-2 in the intestinal mucosa [30]. Then, the increase in bacterial translocation causes gut dysbiosis [30]. Dysregulated autophagy causes gut dysbiosis and vice versa. Targeting gut microbiota would be realistic to decrease dysfunctional autophagy, which in turn would reduce the pathogenic features such as inflammation, oxidative stress, and accumulation of protein aggregates in many neurodegenerative diseases, including AD.6. The Bioflavonoids EGCG and GS as Therapeutic Agents for AD
6.1. Overview of Regulating Cell Signaling by EGCG and GS
Two bioflavonoids, EGCG and GS, have shown major influences on the NF-κB, mitogen-activated protein kinase (MAPK), epidermal growth factor receptor (EGFR), insulin-like growth factor (IGF), and mechanistic target of rapamycin (mTOR) signaling pathways to provide neuroprotection in neurodegenerative diseases (Table 2). Their mechanisms of action on these signaling pathways show their therapeutic efficacies in AD.| Signaling Pathway in AD | Associated Functions | EGCG and GS | References |
|---|---|---|---|
| NF-κB pathway | Regulates pro-inflammatory genes | Both can inhibit the pathway | [29][73][74][75][76] |
| MAPK pathway | Regulates apoptosis, differentiation, etc. | Both can inhibit the pathway | [77][80][81][82][83] |
| EGFR pathway | Regulates gene expression and cell proliferation | Both can inhibit the pathway | [84][85][86][87][88][89][90][91] |
| IGF signal transduction pathway | Regulates cell differentiation, cell survival, and cell maintenance | Both can inhibit the pathway | [91][92][93][94][95][96][97][98][99] |
| mTOR pathway | Regulates cell proliferation, apoptosis, and autophagy | Both can inhibit the pathway | [100][101][102][103][104] |
| 5-Hydroxytryptamine signaling pathway | Regulates serotonin production | Both can facilitate the pathway | [105][106][107][108] |
6.2. Exploration of Bioflavonoids as Therapeutic Options in AD
Through multiple studies, EGCG and GS have been implicated in modulating the signaling pathways related to AD. However, the glaring limitation of using these selective bioflavonoids is their decreased bioavailability. Studies focused on analyzing delivery methods to optimize the absorption and usage of EGCG and GS in the body would be beneficial to develop them as novel therapeutic drugs for the treatment of AD. In this section, multiple methods with goals to administer bioflavonoids to patients are considered: AChE inhibition, diet, fecal microbiota transplantation, neural stem cell therapy, and nanomaterials.6.2.1. AChE Inhibitor
In AD, there is a change in the function of the cholinergic system. The current treatments available for AD mostly provide temporary attenuation of symptoms through cholinergic and anti-glutamatergic mechanisms [109]. Most of the currently used AChE inhibitors have side effects based on the dosage administered. For example, an overdose of Rivastigmine can result in cases of irregular heartbeat and chest pain [110]. Hence, an alternative AChE inhibitor, especially bioflavonoids, would be of interest. An in vitro study showed that neuronal cells treated with 10 µM EGCG reduced Aβ-induced cytotoxicity, with EGCG becoming an AChE inhibitor [111]. Apart from EGCG, GS can function as an efficient AChE inhibitor. In diabetic mice, GS can improve cognitive decline by inhibiting AChE [86]. The basal cholinergic neurons are affected by apolipoprotein E (ApoE) during the pathogenesis of AD. GS upregulates the peroxisome proliferator–activated receptor gamma (PPARγ), which is induced by Aβ deposits. Then, the upregulation of PPARγ results in the production of ApoE, which can decrease the deposition of Aβ [86]. EGCG and GS have the potential to function as AChE and BChE inhibitors, which can replace the current controversial AD therapeutic options on the market.6.2.2. Diet
Dysbiosis, which occurs in the gut due to numerous factors and lack of diversity of the microbiota, can be improved through bioflavonoids, some of which contain antioxidant and antimicrobial properties [112]. However, the issue lies with how exactly EGCG and GS can be made available to patients. The attractive method for the availability of bioflavonoids, apart from these molecules acting as AChE inhibitors, is through diet. The high BBB permeability of EGCG increases neuritogenesis (generation, extension, and diverging of neurites), which attenuates neurodegenerative diseases [113]. If taken along with food, the oral bioavailability of EGCG is low in humans. Studies are showing that if EGCG is taken along with nutrients such as fish oil (omega-3 fatty acids), vitamins (e.g., ascorbic acid), and minerals (e.g., selenium or chromium), then the bioavailability of EGCG improves [113]. Like EGCG, GS also deals with oxidative stress, neuroinflammation, and mitochondrial dysfunction, along with being able to cross BBB to have neuroprotective effects [114]. When the distribution of GS was analyzed, the GS concentration in the GI tract was the highest, followed by the intestine, liver, kidney, lung, heart, brain, reproductive organs, and then muscle [115]. Additionally, the GS concentration in the GI tract was enough to have anti-proliferative effects [115]. Hence, finding ways to properly administer EGCG and GS through a patient’s diet could show changes in the progression of neurodegenerative diseases, including AD.6.2.3. Fecal Microbiota Transplantation (FMT)
Among neurodegenerative diseases, FMT has also shown success in altering dysbiosis in PD patients [116]. In a transgenic mouse model treated with pre-FMT antibiotic treatment to cause dysbiosis, FMT showed the potential to decrease AD pathology [116]. However, FMT from young mice had more significant changes compared to microbiota from aged mice, which resulted in chronic low-grade inflammation or imflammaging. After the FMT in AD mice, the BBB and the metabolite levels were repaired, allowing for attenuation of AD pathogenesis [116]. Bioflavonoids can also help increase the advantages of FMT in diseases. In a study reported in 2021, FMT was conducted using microbiota from EGCG-dosed mice [117]. The results showed that microbiota retrieved from EGCG-dosed mice decreased inflammation and developed the colonic barrier integrity along with producing SCFAs and effective bacteria such as Akkermansia [117], also a commensal (neither harmful nor beneficial) microbe making up 1–4% of gut microbes in humans. On a similar note, microbiota from GS-dosed mice increased SCFA production and revived the gut flora, allowing the recipient mice to live longer [118]. The efficiency of FMT with EGCG and GS should be further explored for administering them as an alternative therapeutic strategy for AD patients.6.2.4. Neural Stem Cell Therapy
Neural stem cells (NSCs) are pluripotent stem cells that exist solely in the CNS. NSCs can proliferate and differentiate into multiple cell types (e.g., neurons, oligodendrocytes, and astrocytes) [119][120]. Cellular therapy makes use of neurogenic or non-neurogenic cells to improve nerve repair and tissue damage, and this method has been widely used to treat CNS diseases [120]. Neural stem cell therapy uses a mechanism of regulating the local microenvironment, increasing blood vessel development and neuron regeneration, and attenuating inflammatory responses [120]. A mice model study used NSCs obtained from the fetal brain tissue, showing the hippocampus of the recipient 3xTg-AD mice improving cognitively through enhanced endogenous synaptogenesis [121]. Further studies have shown human brain-derived NSCs (hNSCs) injected into the hippocampus of APP/PSEN1 model of AD, resulting in a development of neuronal connectivity and metabolic activity, which allowed for a decrease in AD pathogenesis [122]. Other than attenuating cognitive defects, NSCs can also inhibit inflammatory responses, neuronal loss, and regulation of microglia function [123][124]. Tea polyphenols can increase the survival rate of NSCs based on the concentration administered [125]. A study focused on the differentiation of NSCs from mouse cochlear (a fluid-filled, spiral cavity in the inner ear) in which researchers found EGCG stimulating the proliferation and neurosphere formation in the isolated NSCs in vitro [126]. In a study, ischemic stroke was induced in NPCs, and the effect of EGCG in vitro and in vivo was analyzed. After 14 days of treatment with EGCG, the neuronal differentiation was increased in the cultured NPCs [127]. Unfortunately, there are no studies with GS, specifically regarding NSCs. However, a study was conducted in which GS and daidzein (an isoflavone found exclusively in soybeans and other legumes) increased the hippocampus neuronal cell viability and proliferation in vitro [128]. Further studies should be explored to understand the influence of GS on NSC therapy for AD patients. A combination of EGCG and GS could further promote the proliferation and differentiation of NSCs in the treatment of AD.6.2.5. Nanomaterials
The major issue with administering polyphenols is their low bioavailability. The reasoning can be attributed to intrinsic factors (e.g., chemical structure, molecular weight, and low hydro solubility or solubility in water) and extrinsic factors (e.g., low stability in the GI tract, extensive Phase I and Phase II metabolism, and rapid elimination [129]. A solution to combat this low bioavailability is using polymeric nanoparticle-based delivery systems, which deliver bioactive molecules across the GI tract to target organs [129]. A nanoparticle refers to a small particle that ranges between 1 to 100 nm in size [130]. There are a variety of nanoparticle systems, such as nanospheres (NSs), nanocapsules (NCs), solid lipid nanoparticles (SLNs), cyclodextrins (CDs), liposomes (LSs), and micelles (MCs). Specifically, for polyphenols, biodegradable and biocompatible polymers are the most explored as a nanoparticle system [129]. Existing studies confirm the possibility of delivering EGCG and GS with different nanoparticles. EGCG was loaded into heat-treated β-lactoglobulin (β-Lg), which stabilizes the structure of the bioflavonoid and helps protect its antioxidant properties [131]. Further studies reaffirm the advantages of using nanomaterials to deliver EGCG. The absorption of EGCG in the GI tract can be improved by using chitosan/trimeric phosphate nanoparticles [132]. With the bioavailability of EGCG increased orally, the amount of EGCG available to plasma and jejunum also increased [132]. Shifting the focus to GS regarding clinical applications, which also have rapid metabolism and excretion, and low oral bioavailability [133]. The mucoadhesive polymers can reside in the nasal pathway longer, regulating drug release and intracellular uptake [133]. The results regarding the efficacy of using EGCG and GS in tandem with nanoparticles look positive and highly promising for the treatment of AD; hence, this is a therapeutic drug delivery method that should be further explored in detail for clinical trials.References
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789.
- Graff-Radford, J.; Yong, K.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234.
- U.S. Department of Health and Human Services. Alzheimer’s Disease Fact Sheet. National Institute on Aging. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet#:~:text=Alzheimer%27s%20disease%20is%20a%20brain,first%20appear%20later%20in%20life (accessed on 20 May 2023).
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 167, pp. 231–255.
- Landau, S.M.; Lu, M.; Joshi, A.D.; Pontecorvo, M.; Mintun, M.A.; Trojanowski, J.Q.; Shaw, L.M.; Jagust, W.J. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol. 2013, 74, 826–836.
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and Diagnostic Criteria. Alzheimers Dement. 2016, 12, 292–323.
- Alzheimer’s Association. Medications for Memory, Cognition and Dementia-Related Behaviors. Alzheimer’s Disease and Dementia. Available online: https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory (accessed on 20 May 2023).
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host Inflammasome Influence Brain Physiology and pathology. Front. Immunol. 2020, 11, 604179.
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (review). Mol. Med. Rep. 2019, 20, 1479–1487.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346.
- De Bruyne, T.; Steenput, B.; Roth, L.; De Meyer, G.; Santos, C.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary polyphenols targeting arterial stiffness: Interplay of contributing mechanisms and gut microbiome-related metabolism. Nutrients 2019, 11, 578.
- WebMD. 10 Foods High in Flavonoids and Why You Need Them. 2023. Available online: https://www.webmd.com/diet/foods-high-in-flavonoids (accessed on 20 May 2023).
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57.
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for alzheimer disease. Free. Radic. Biol. Med. 2012, 52, 35–45.
- Narayana, R.K.; Reddy, S.M.; Chaluvadi, M.R.; Krishna, D.R. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16.
- Zhang, Z.; Zhang, Y.; Li, J.; Fu, C.; Zhang, X. The neuroprotective effect of tea polyphenols on the regulation of intestinal flora. Molecules 2021, 26, 3692.
- Hole, K.L.; Williams, R.J. Flavonoids as an intervention for Alzheimer’s disease: Progress and hurdles towards defining a mechanism of Action1. Brain Plast. 2021, 6, 167–192.
- Roseiro, L.B.; Rauter, A.P.; Serralheiro, M.L. Polyphenols as acetylcholinesterase inhibitors: Structural specificity and impact on human disease. Nutr. Aging 2012, 1, 99–111.
- Potenza, M.A.; Iacobazzi, D.; Sgarra, L.; Montagnani, M. The intrinsic virtues of EGCG, an extremely good cell guardian, on prevention and treatment of diabesity complications. Molecules 2020, 25, 3061.
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022, 13, 820969.
- Hong, M.; Zhang, R.; Liu, Y.; Wu, Z.; Weng, P. The interaction effect between tea polyphenols and intestinal microbiota: Role in ameliorating neurological diseases. J. Food Biochem. 2021, 46, e13870.
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An integrative overview of its mode of action, pharmacological properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota. Diabetes Care 2010, 33, 2277–2284.
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, its role in induction of Alzheimer’s disease pathology, and possible therapeutic interventions: Special focus on anthocyanins. Cells 2020, 9, 853.
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J. Parenter. Enter. Nutr. 2020, 45, 518–529.
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2021, 231, 107988.
- Martínez-Cerdeño, V.; Noctor, S.C. Neural progenitor cell terminology. Front. Neuroanat. 2018, 12, 104.
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2019, 154, 635–646.
- WebMD. Dysbiosis: Gut Imbalance, IBD, and More. WebMD, 2022. Available online: https://www.webmd.com/digestive-disorders/what-is-dysbiosis (accessed on 22 May 2023).
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150.
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol. Neurobiol. 2020, 57, 5026–5043.
- Wisniewski, P.J.; Dowden, R.A.; Campbell, S.C. Role of dietary lipids in modulating inflammation through the gut microbiota. Nutrients 2019, 11, 117.
- Nyangale, E.P.; Mottram, D.S.; Gibson, G.R. Gut microbial activity, implications for health and disease: The potential role of metabolite analysis. J. Proteome Res. 2012, 11, 5573–5585.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871.
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512.
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.-H. Crosstalk between gut and brain in Alzheimer’s disease: The role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690.
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L.; et al. Altered Gut Microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2017, 60, 1241–1257.
- Cho, J.; Park, Y.J.; Gonzales-Portillo, B.; Saft, M.; Cozene, B.; Sadanandan, N.; Borlongan, C.V. Gut dysbiosis in stroke and its implications on Alzheimer’s disease-like cognitive dysfunction. CNS Neurosci. Ther. 2021, 27, 505–514.
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 953718.
- Huynh, V.A.; Takala, T.M.; Murros, K.E.; Diwedi, B.; Saris, P.E. Desulfovibrio bacteria enhance alpha-synuclein aggregation in a Caenorhabditis elegans model of parkinson’s disease. Front. Cell. Infect. Microbiol. 2023, 13, 502.
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028.
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in gut microbiota composition in an app/pss1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471.
- Poole, S.; Singhrao, S.K.; Chukkapalli, S.; Rivera, M.; Velsko, I.; Kesavalu, L.; Crean, S. Active invasion of porphyromonas gingivalis and infection-induced complement activation in apoe-/- mice brains. J. Alzheimers Dis. 2014, 43, 67–80.
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68.
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405.
- Waldstein, S.R.; Wendell, C.R.; Seliger, S.L.; Ferrucci, L.; Metter, E.J.; Zonderman, A.B. Nonsteroidal anti-inflammatory drugs, aspirin, and cognitive function in the Baltimore Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2010, 58, 38–43.
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to Better Brain Health. perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80.
- Mira-Pascual, L.; Cabrera-Rubio, R.; Ocon, S.; Costales, P.; Parra, A.; Suarez, A.; Moris, F.; Rodrigo, L.; Mira, A.; Collado, M.C. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J. Gastroenterol. 2014, 50, 167–179.
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health 2020, 17, 7618.
- Mohr, A.E.; Crawford, M.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: Considering structural variation. FEBS Lett. 2022, 596, 849–875.
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832.
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 8243–8250.
- Watanabe, D.; Guo, Y.; Kamada, N. Interaction between the inflammasome and commensal microorganisms in gastrointestinal health and disease. EMBO Mol. Med. 2021, 13, e13452.
- Yan, Y.-Q.; Ma, C.-G.; Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710.
- Di Sabatino, A.; Cazzola, P.; Ciccocioppo, R.; Morera, R.; Biancheri, P.; Rovedatti, L.; Cantoro, L.; Vanoli, A.; Tinozzi, F.P.; Tinozzi, S.; et al. Efficacy of butyrate in the treatment of mild to moderate Crohn’s disease. Dig. Liver Dis. Suppl. 2007, 1, 31–35.
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687.
- Man, S.M. Inflammasomes in the gastrointestinal tract: Infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 721–737.
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The role of NLRP3 inflammasome in Alzheimer’s disease and potential therapeutic targets. Front. Pharmacol. 2022, 13, 845185.
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 2022, 14, 879021.
- Barczuk, J.; Siwecka, N.; Lusa, W.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. Targeting NLRP3-mediated neuroinflammation in Alzheimer’s disease treatment. Int. J. Mol. Sci. 2022, 23, 8979.
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678.
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O’Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in app/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316.
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673.
- Rao, S.; Schieber, A.M.; O’Connor, C.P.; Leblanc, M.; Michel, D.; Ayres, J.S. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell 2017, 168, 503–516.e12.
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the gut-microbial-inflammasome-brain axis in a mouse model of Alzheimer’s disease. Cells 2021, 10, 779.
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hao, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 100, 109884.
- Zhang, Z.; Yang, X.; Song, Y.-Q.; Tu, J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res. Rev. 2021, 72, 101464.
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s disease: From molecular mechanisms to therapeutic implications. Front. Aging Neurosci. 2018, 10, 4.
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12.
- Manea, A.J.; Ray, S.K. Regulation of autophagy as a therapeutic option in glioblastoma. Apoptosis 2021, 26, 574–599.
- Zare-shahabadi, A.; Masliah, E.; Johnson, G.V.W.; Rezaei, N. Autophagy in Alzheimer’s disease. Rev. Neurosci. 2015, 26, 385–395.
- Wang, L.; Klionsky, D.J.; Shen, H.-M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2022, 24, 186–203.
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381.
- Wong, A.S.L.; Cheung, Z.H.; Ip, N.Y. Molecular machinery of macroautophagy and its deregulation in diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1490–1497.
- Lapaquette, P.; Bizeau, J.-B.; Acar, N.; Bringer, M.-A. Reciprocal interactions between gut microbiota and autophagy. World J. Gastroenterol. 2021, 27, 8283–8301.
- Shoubridge, A.P.; Fourrier, C.; Choo, J.M.; Proud, C.G.; Sargeant, T.J.; Rogers, G.B. Gut microbiome regulation of autophagic flux and Neurodegenerative Disease Risks. Front. Microbiol. 2021, 12, 817433.
- Nixon, R.A. The role of autophagy in Neurodegenerative Disease. Nat. Med. 2013, 19, 983–997.
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505.
- Joo, S.-Y.; Song, Y.-A.; Park, Y.-L.; Myung, E.; Chung, C.-Y.; Park, K.-J.; Cho, S.-B.; Lee, W.-S.; Kim, H.-S.; Rew, J.-S.; et al. Epigallocatechin-3-gallate inhibits LPS-induced NF-ΚB and MAPK signaling pathways in bone marrow-derived macrophages. Gut Liver 2012, 6, 188–196.
- Jiang, J.; Mo, Z.-C.; Yin, K.; Zhao, G.-J.; Lv, Y.-C.; Ouyang, X.-P.; Jiang, Z.-S.; Fu, Y.; Tang, C.-K. Epigallocatechin-3-gallate prevents TNF-α-induced NF-ΚB activation thereby upregulating ABCA1 via the nrf2/KEAP1 pathway in macrophage foam cells. Int. J. Mol. Med. 2012, 29, 946–956.
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the neuroprotective effects of genistein on Alzheimer’s disease. Brain Behav. 2021, 11, e02100.
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254.
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18.
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. Erk/MAPK signalling pathway and tumorigenesis (review). Exp. Ther. Med. 2020, 19, 1997–2007.
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic effects of green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2022, 24, 340.
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F. Epigallocatechin-3-gallate (EGCG): New therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules 2022, 12, 371.
- Xu, D.; Peng, S.; Guo, R.; Yao, L.; Mo, H.; Li, H.; Song, H.; Hu, L. EGCG alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via MAPK signaling pathway. Toxins 2021, 13, 693.
- Uddin, M.S.; Kabir, M.T. Emerging signal regulating potential of genistein against Alzheimer’s disease: A promising molecule of interest. Front. Cell Dev. Biol. 2019, 7, 197.
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52.
- Creative Diagnostics. EGF/EGFR Signaling Pathway. Available online: https://www.creative-diagnostics.com/egf-egfr-signaling-pathway.htm#:~:text=EGFR%20signaling%20pathway%20is%20one,regulate%20intercellular%20communication%20during%20development (accessed on 15 June 2023).
- Yarden, Y.; Shilo, B.Z. SnapShot: EGFR signaling pathway. Cell 2007, 131, 1018.
- Mansour, H.M.; Fawzy, H.M.; El-Khatib, A.S.; Khattab, M.M. Repurposed anti-cancer epidermal growth factor receptor inhibitors: Mechanisms of neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1913–1918.
- Ettcheto, M.; Cano, A.; Sanchez-López, E.; Verdaguer, E.; Folch, J.; Auladell, C.; Camins, A. Masitinib for the treatment of Alzheimer’s disease. Neurodegener. Dis. Manag. 2021, 11, 263–276.
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of epigallocatechin-3-gallate on EGFR signaling and migration in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 11833.
- Farabegoli, F.; Govoni, M.; Spisni, E.; Papi, A. EGFR inhibition by (-)-epigallocatechin-3-gallate and IIF treatments reduces breast cancer cell invasion. Biosci. Rep. 2017, 37, BSR20170168.
- Mas-Bargues, C.; Borrás, C.; Viña, J. The multimodal action of genistein in Alzheimer’s and other age-related diseases. Free. Radic. Biol. Med. 2022, 183, 127–137.
- Hakuno, F.; Takahashi, S.-I. 40 years of IGF1: IGF1 receptor signaling pathways. Mol. Endocrinol. 2018, 61, T69–T86.
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64.
- Westwood, A.J.; Beiser, A.; DeCarli, C.; Harris, T.B.; Chen, T.C.; He, X.-M.; Roubenoff, R.; Pikula, A.; Au, R.; Braverman, L.E.; et al. Insulin-like growth factor-1 and risk of alzheimer dementia and brain atrophy. Neurology 2014, 82, 1613–1619.
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008, 262, 10–18.
- Sakai, H.; Shimizu, M.; Shirakami, Y.; Weinstein, I.; Moriwaki, H. Effects of EGCG on activation of the IGF/IGF-1R system in human hepatoma cells. Cancer Res. 2007, 67 (Suppl. S9), 1573. Available online: https://aacrjournals.org/cancerres/article/67/9_Supplement/1573/535894/Effects-of-EGCG-on-activation-of-the-IGF-IGF-1R (accessed on 20 May 2023).
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/P-akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000.
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31.
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25.
- Rogers, S.L.; Farlow, M.R.; Doody, R.S.; Mohs, R.; Friedhoff, L.T. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998, 50, 136–145.
- Okello, E.J.; Leylabi, R.; McDougall, G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012, 3, 651–661.
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease. Front. Nutr. 2021, 8, 798038.
- Andreu Fernández, V.; Almeida Toledano, L.; Pizarro Lozano, N.; Navarro Tapia, E.; Gómez Roig, M.D.; De la Torre Fornell, R.; García Algar, Ó. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers. Antioxidants 2020, 9, 440.
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A focus on several neurodegenerative diseases. J. Food Biochem. 2022, 46, e14155.
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of Genistein: Mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280.
- Elangovan, S.; Borody, T.J.; Holsinger, R.M. Fecal microbiota transplantation reduces pathology and improves cognition in a mouse model of Alzheimer’s disease. Cells 2022, 12, 119.
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184.
- Hou, Q.; Huang, J.; Zhao, L.; Pan, X.; Liao, C.; Jiang, Q.; Lei, J.; Guo, F.; Cui, J.; Guo, Y.; et al. Dietary genistein increases microbiota-derived short chain fatty acid levels, modulates homeostasis of the aging gut, and extends healthspan and lifespan. Pharmacol. Res. 2023, 188, 106676.
- Zhao, L.; Liu, J.-W.; Shi, H.-Y.; Ma, Y.-M. Neural stem cell therapy for brain disease. World J. Stem Cells 2021, 13, 1278–1292.
- Kim, H.-J. Regulation of neural stem cell fate by natural products. Biomol. Ther. 2019, 27, 15–24.
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826.
- Li, X.; Zhu, H.; Sun, X.; Zuo, F.; Lei, J.; Wang, Z.; Bao, X.; Wang, R. Human neural stem cell transplantation rescues cognitive defects in App/PS1 model of Alzheimer’s disease by enhancing neuronal connectivity and metabolic activity. Front. Aging Neurosci. 2016, 8, 282.
- Ryu, J.K.; Cho, T.; Wang, Y.T.; McLarnon, J.G. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed ad brain. J. Neuroinflamm. 2009, 6, 39.
- De Almeida, M.M.; Goodkey, K.; Voronova, A. Regulation of microglia function by neural stem cells. Front. Cell. Neurosci. 2023, 17, 1130205.
- Cheng, Y.; Sun, J.; Zhao, H.; Guo, H.; Li, J. Functional mechanism on stem cells by tea (Camellia sinensis) bioactive compounds. Food Sci. Hum. Wellness 2022, 11, 579–586.
- Zhang, Y.; He, Q.; Dong, J.; Jia, Z.; Hao, F.; Shan, C. Effects of epigallocatechin-3-gallate on proliferation and differentiation of mouse cochlear neural stem cells: Involvement of PI3K/akt signaling pathway. Eur. J. Pharm. Sci. 2016, 88, 267–273.
- Zhang, J.-C.; Xu, H.; Yuan, Y.; Chen, J.-Y.; Zhang, Y.-J.; Lin, Y.; Yuan, S.-Y. Delayed treatment with green tea polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol. Neurobiol. 2016, 54, 3652–3664.
- Pan, M.; Han, H.; Zhong, C.; Geng, Q. Effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression in H19-7 neural cell line. J. Nutr. Health Aging 2011, 16, 389–394.
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M.A.B. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317.
- TWI. What are Nanoparticles? Definition, Size, Uses and Properties. Available online: https://www.twi-global.com/technical-knowledge/faqs/what-are-nanoparticles (accessed on 4 August 2023).
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484.
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A Review. J. Funct. Foods 2020, 65, 103732.
- Rassu, G.; Porcu, E.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics 2018, 11, 8.
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021, 21, 388.
- Sahu, A.; Gopalakrishnan, L.; Gaur, N.; Chatterjee, O.; Mol, P.; Modi, P.K.; Dagamajalu, S.; Advani, J.; Jain, S.; Keshava Prasad, T.S. The 5-hydroxytryptamine signaling map: An overview of serotonin-serotonin receptor mediated signaling network. J. Cell Commun. Signal. 2018, 12, 731–735.
- Uceda, S.; Echeverry-Alzate, V.; Reiriz-Rojas, M.; Martínez-Miguel, E.; Pérez-Curiel, A.; Gómez-Senent, S.; Beltrán-Velasco, A.I. Gut microbial metabolome and dysbiosis in neurodegenerative diseases: Psychobiotics and fecal microbiota transplantation as a therapeutic approach—A comprehensive narrative review. Int. J. Mol. Sci. 2023, 24, 13294.
- Dunham, S.J.; McNair, K.A.; Adams, E.D.; Avelar-Barragan, J.; Forner, S.; Mapstone, M.; Whiteson, K.L. Longitudinal analysis of the microbiome and metabolome in the 5xfad mouse model of Alzheimer’s disease. mBio 2022, 13, e0179422.
- Li, G.; Yang, J.; Wang, X.; Zhou, C.; Zheng, X.; Lin, W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020, 11, 8780–8787.
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 2019, 24, 3892.
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25.
- Kim, J.-W.; Im, S.; Jeong, H.-R.; Jung, Y.-S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.-O. Neuroprotective effects of Korean red pine (pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687.
- Okello, E.J.; Mather, J. Comparative kinetics of acetyl- and butyryl-cholinesterase inhibition by green tea catechins|relevance to the symptomatic treatment of Alzheimer’s disease. Nutrients 2020, 12, 1090.
- Okello, E.J.; Leylabi, R.; McDougall, G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012, 3, 651–661.
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease. Front. Nutr. 2021, 8, 798038.
- Andreu Fernández, V.; Almeida Toledano, L.; Pizarro Lozano, N.; Navarro Tapia, E.; Gómez Roig, M.D.; De la Torre Fornell, R.; García Algar, Ó. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers. Antioxidants 2020, 9, 440.
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A focus on several neurodegenerative diseases. J. Food Biochem. 2022, 46, e14155.
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of Genistein: Mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280.
- Pamer, E.G. Fecal microbiota transplantation: Effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014, 7, 210–214.
- Cheng, S.; Ma, X.; Geng, S.; Jiang, X.; Li, Y.; Hu, L.; Li, J.; Wang, Y.; Han, X. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury. mSystems 2018, 3, e00137-18.
- Zhang, X.; Ishikawa, D.; Ohkusa, T.; Fukuda, S.; Nagahara, A. Hot topics on fecal microbiota transplantation for the treatment of inflammatory bowel disease. Front. Med. 2022, 9, 106856.
- Elangovan, S.; Borody, T.J.; Holsinger, R.M. Fecal microbiota transplantation reduces pathology and improves cognition in a mouse model of Alzheimer’s disease. Cells 2022, 12, 119.
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184.
- Hou, Q.; Huang, J.; Zhao, L.; Pan, X.; Liao, C.; Jiang, Q.; Lei, J.; Guo, F.; Cui, J.; Guo, Y.; et al. Dietary genistein increases microbiota-derived short chain fatty acid levels, modulates homeostasis of the aging gut, and extends healthspan and lifespan. Pharmacol. Res. 2023, 188, 106676.
- Zhao, L.; Liu, J.-W.; Shi, H.-Y.; Ma, Y.-M. Neural stem cell therapy for brain disease. World J. Stem Cells 2021, 13, 1278–1292.
- Kim, H.-J. Regulation of neural stem cell fate by natural products. Biomol. Ther. 2019, 27, 15–24.
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826.
- Li, X.; Zhu, H.; Sun, X.; Zuo, F.; Lei, J.; Wang, Z.; Bao, X.; Wang, R. Human neural stem cell transplantation rescues cognitive defects in App/PS1 model of Alzheimer’s disease by enhancing neuronal connectivity and metabolic activity. Front. Aging Neurosci. 2016, 8, 282.
- Ryu, J.K.; Cho, T.; Wang, Y.T.; McLarnon, J.G. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed ad brain. J. Neuroinflamm. 2009, 6, 39.
- De Almeida, M.M.; Goodkey, K.; Voronova, A. Regulation of microglia function by neural stem cells. Front. Cell. Neurosci. 2023, 17, 1130205.
- Cheng, Y.; Sun, J.; Zhao, H.; Guo, H.; Li, J. Functional mechanism on stem cells by tea (Camellia sinensis) bioactive compounds. Food Sci. Hum. Wellness 2022, 11, 579–586.
- Zhang, Y.; He, Q.; Dong, J.; Jia, Z.; Hao, F.; Shan, C. Effects of epigallocatechin-3-gallate on proliferation and differentiation of mouse cochlear neural stem cells: Involvement of PI3K/akt signaling pathway. Eur. J. Pharm. Sci. 2016, 88, 267–273.
- Zhang, J.-C.; Xu, H.; Yuan, Y.; Chen, J.-Y.; Zhang, Y.-J.; Lin, Y.; Yuan, S.-Y. Delayed treatment with green tea polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol. Neurobiol. 2016, 54, 3652–3664.
- Pan, M.; Han, H.; Zhong, C.; Geng, Q. Effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression in H19-7 neural cell line. J. Nutr. Health Aging 2011, 16, 389–394.
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M.A.B. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317.
- TWI. What are Nanoparticles? Definition, Size, Uses and Properties. Available online: https://www.twi-global.com/technical-knowledge/faqs/what-are-nanoparticles (accessed on 4 August 2023).
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484.
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A Review. J. Funct. Foods 2020, 65, 103732.
- Rassu, G.; Porcu, E.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics 2018, 11, 8.
