2. Introduction to microRNA
MiRNAs are a class of endogenous, single-stranded, noncoding ribonucleic acid molecules found in eukaryotic cells
[12][15]. They are about 19 to 25 nucleotides in length and function to regulate post-transcriptional gene expression by binding to the 3′UTR of target mRNA to suppress or enhance its translation
[12][15]. Binding to 5′UTR, gene promoters and coding sequences have also been described, suggesting an effect on both translation and transcription
[13][14][16,17]. MiRNAs are generally divided into two classes according to their origin in the genome: those encoded in exons and those from overlapping introns, with around 50% being expressed from non-protein coding regions
[15][18]. The expression of miRNA is affected by the same regulatory mechanisms of protein-coding genes, such as epigenetic regulation and a regulatory feedback loop
[16][17][19,20]. Growing evidence suggests that these small molecules play a pivotal role in cell differentiation and proliferation, metabolism, apoptosis, stress responses, and cell-cycle regulation
[18][19][21,22].
MiRNAs are processed via canonical or non-canonical pathways, the canonical pathway being predominant
[14][15][17,18]. Key proteins involved in miRNA biogenesis in both pathways include the Drosha, Dicer, exportin-5, and Argonaute family proteins
[14][17]. In the canonical pathway, the synthesis of miRNA is initiated in the nucleus by the binding of RNA polymerase II/III to DNA genes encoding for primary miRNA (pri-miRNA), either post- or co-transcriptionally
[14][17]. A stem-loop structure of pri-miRNA is transcribed, which is then cleaved into a hairpin-structure precursor miRNA (pre-miRNA) by the enzyme Drosha. Pre-miRNA is transported to the cytoplasm through the exportin-5 complex to be processed by the enzyme Dicer-transactivation response RNA binding protein (TRBP) into a miRNA duplex
[20][23]. Further structural processing of the miRNA duplex occurs to produce a mature miRNA
[14][15][17,18]. Understanding these processes is crucial to developing therapeutic strategies targeting miRNAs
[21][22][24,25].
Moreover, the study of miRNAs has provided a new perspective on the regulation of gene expression post-transcriptionally
[23][26]. Abnormal miRNA expression has been associated with several human diseases, such as type 2 diabetes, obesity, cardiovascular disease, PCOS, endometriosis, and cancer
[17][24][25][26][27][20,27,28,29,30]. In addition to cellular expression, miRNAs can also be found circulating in the plasma and serum
[28][31]. They have also been detected in urine, semen, saliva, and microvesicles, making them useful potential diagnostic and prognostic markers of disease
[16][19]. MiRNAs can be secreted outside cells, loaded into high-density lipoproteins, attached to Argonaute RISC catalytic component 2 (Ago2), or packaged in exosomes
[23][26]. The release of exosomal miRNA in extracellular fluid is mediated by a ceramide-dependent pathway and is thought to have a growth regulatory effect on target cells
[29][32].
Furthermore, miRNAs have been suggested to play a role in the onset and development of PCOS
[30][31][33,34]. Under normal conditions, they are expressed in theca cells, follicular fluid, and granulosa cells and are considered important regulators of metabolic processes
[30][33]. Key pathways regulated by miRNA include folliculogenesis, steroidogenesis, and cellular adhesion, each of which is hypothesized to be involved in the pathogenesis of PCOS
[30][33]. In PCOS women, miRNAs were found to be abnormally expressed in theca cells, follicular fluid, granulosa cells, peripheral blood leukocytes, serum, and adipose tissue when compared to women without PCOS
[30][33]. As such, miRNAs have been widely studied with the aim of understanding the pathogenesis of PCOS and identifying potential biomarkers for the disease
[31][34].
3. The Role of miRNA in the Pathogenesis of Common PCOS Complications
3.1. Infertility
PCOS is characterized by ovulatory dysfunction, making it more likely for patients to experience poor pregnancy outcomes. Women suffering from PCOS experience an alarming rate of infertility and are at risk of fetal, neonatal, and maternal complications
[32][33][35,36]. The incidence of PCOS in women with anovulatory infertility is 70–80%
[34][37]. Unsurprisingly, a significant number of PCOS women will undergo infertility treatment in order to conceive, with many commonly experiencing pregnancy complications such as pre-eclampsia, premature birth, and gestational diabetes
[31][35][34,38]. Moreover, it is believed that there is an association between insulin resistance (IR) and hyperandrogenism that is directly linked to infertility problems in PCOS
[36][40] (
Figure 1).
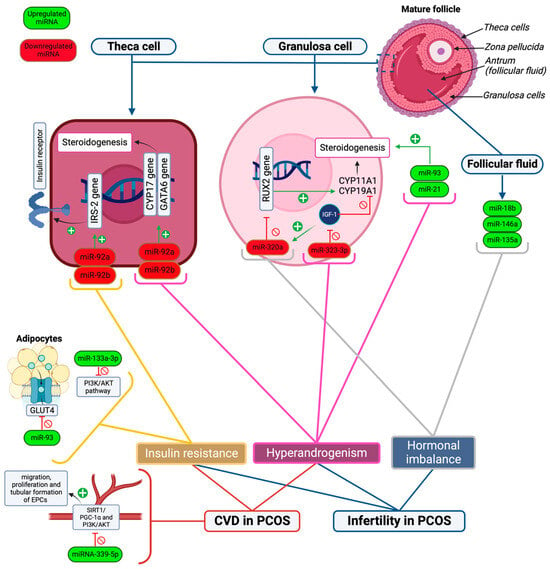
Figure 1. A simplified model showcasing the role of important miRNAs in the pathogenesis of PCOS complications. Upregulated miRNAs are shown in green font, and downregulated miRNAs are shown in red font. The main drivers of PCOS complications are insulin resistance, hyperandrogenism, and other hormonal imbalances. Infertility in PCOS is mediated by insulin resistance, hyperandrogenism, and hormonal imbalance, while CVD risk is affected by hyperandrogenism, insulin resistance, and reduced vascular endothelial health. The figure shows that the inhibition of the PI3K/AKT pathway in cells (due to the up regulation of miR-133a-3p), GLUT4 receptors (due to upregulation of miR-93), and increased expression of IRS-2 proteins (due to downregulation of miR-92a and -92b) play a role in insulin resistance in PCOS. Meanwhile, the downregulation of miR-323-3p, which reduces the activity of IGF-1, and upregulation of miR-93 and -21 in granulosa cells results in hyperandrogenism. Likewise, the downregulation of miR-92a and -92b in theca cells leads to hyperandrogenism by increasing the expression of CYP17 and GATA6 genes which stimulate androgen synthesis. Moreover, dysregulated levels of progesterone, estradiol, and testosterone are affected by the upregulation of miR-18b, -146a, and -135a in follicular fluid, resulting in hormonal imbalance. The downregulation of miR-320a also leads to hormonal variations by reducing the expression of the RUX2 gene, which modulates steroidogenesis in granulosa cells. Additionally, miRNA-339-5p leads to vascular endothelial cell damage by inhibiting SIRT1/PGC-1α and PI3K/AKT signaling pathways, which inhibits EPC migration, tubular formation, and proliferation and contributes to the risk of CVD in PCOS.
Various microRNAs are significantly increased in PCOS women, causing hormonal imbalances that disrupt the menstrual cycle. Three of these microRNAs were identified to be overexpressed in the follicular fluid of PCOS patients compared to healthy controls. This includes miR-18b, miR-146a, and miR-135a
[37][41]. MiR-18b promotes the release of progesterone while inhibiting the release of estradiol and testosterone, thus negatively impacting fertility. MiR-146a reduces progesterone, estradiol, and testosterone release, causing irregularities in the menstrual cycle. MiR-135a reduces progesterone and testosterone release
[37][41].
It has also been demonstrated that hyperandrogenic PCOS patients have increased expression of miR-93 and miR-21 in comparison to normal androgenic PCOS patients. MiR-93 and miR-21 have been highlighted as androgen-dependent factors, as free androgen and free testosterone index positively correlated with them in granulosa cells of women with PCOS. This indicates that, under hyperandrogenic conditions, they may have a role in follicular dysfunction
[38][42]. In addition, miRNAs have been identified as having an impact on relative estrogen deficiency. A significant imbalance in estradiol and androgen in granulosa cells has been related to miR-27a-3p
[39][43]. Additionally, the downregulation of miR-320a in granulosa cells is thought to promote estrogen deficiency and impact insulin-like growth factor 1 (IGF-1) regulatory mechanisms
[40][44]. This is mediated through the expression of CYP11A1 and CYP19A1 by direct targeting of the
RUNX2 gene
[40][44].
MiRNAs also play an important role in ovarian theca cell function. It has been suggested that PCOS patients have downregulated expression of miR-92a and miR-92b in theca cells, which regulate 17-hydroxylase/C17–20 lyase cytochrome P50 (
CYP17), GATA-binding factor 6 (
GATA6), and insulin receptor substrate proteins 2 (IRS-2) expression
[41][45]. This could play a role in androgen biosynthesis dysregulation in theca cells, as theca cells and granulosa cells usually produce non-steroidal factors that affect mutual differentiation and proliferation throughout folliculogenesis
[20][41][23,45]. Moreover, miR-323-3p found in cumulus cells has also been proven to be dysregulated in PCOS patients compared to healthy controls
[39][43]. It is thought that miR-323-3p directly binds to IGF-1 mRNA and inhibits steroidogenesis and cumulus cell apoptosis in women who do not suffer from PCOS. However, in PCOS patients, miR-323-3p is downregulated, leading to the upregulation of steroidogenesis and promotion of apoptosis, which indicates that it could have a significant role in the development of PCOS and infertility
[39][43].
Overall, existing evidence supports an association between PCOS and miRNA dysregulation in granulosa cells and theca cells, which may contribute to PCOS evolution, hyperandrogenemia, and complications with regard to fertility. This implies that manipulation of miRNAs could have a role in improving follicular status and health in women with PCOS.
3.2. Insulin Resistance
Insulin resistance (IR) in PCOS is caused by impaired insulin action characterized by compensatory hyperinsulinemia (HI) and reduced insulin response to glucose overload. As such, IR in women alters several metabolic pathways in target organs, including, but not limited to, adipose tissue, skeletal muscle, liver, and brain. Androgen excess promotes a vicious cycle with IR and HI
[42][46]. IR and compensatory HI are present in 65–95% of women diagnosed with PCOS and are exacerbated by obesity
[42][46].
In women with PCOS, some miRNAs are increased in expression and associated with insulin resistance. One such miRNA is miRNA-93, which was shown to be overexpressed and to correlate strongly with the downregulation of GLUT4 receptors in adipose tissue in women with PCOS
[43][47]. However, it was also observed to be elevated in other IR-associated conditions such as Type 2 diabetes mellitus (T2DM) and obesity
[43][47]. Another key miRNA is miRNA-146, which is closely associated with insulin resistance and is believed to play a key role in IR pathogenesis. Expression of this miRNA leads to a cascade of proinflammatory signals in which nuclear factor kappa B (NFkB) becomes activated, which increases miR-146a levels in order to institute negative feedback regulation and control of immune responses. During hyperglycemia, miR-146 levels may be decreased despite NFkB activation and proinflammatory responses, which could originate from pre-miR-146 polymorphisms
[44][48]. Moreover, this miRNA could be useful as a diagnostic or therapeutic target, but this requires further investigation
[44][48]. MiRNA-133a-3p was also more highly expressed in PCOS women compared to controls, with a further study demonstrating that, amongst PCOS subjects, obese PCOS women had the highest expression levels
[44][48]. This miRNA inhibits the phosphoinositide-3-kinase/protein kinase B (PI3K/AKT(PKB)) signaling pathway, which is well-established for its importance in insulin action. Insulin activates PI3K through the insulin receptor substrate-1 to promote its main downstream molecular protein, AKT, which is activated by PI3K phosphorylation on ser473 and thr308. IR in PCOS leads to PI3K/AKT signaling inhibition. However, further studies are required to fully elucidate the mechanisms involved
[44][48].
Furthermore, the administration of metformin has been linked with the altered expression of miR-222 and miR-221 in patients with T2DM
[45][49]. Studies on dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide 1 agonist receptor agonist (GLP-1 RA) noted a similar relationship, including the altered expression of miR-6763, miR-33, miR-155-5p, miR-6356, miR-197, miR-875-5P, and miR-1197-3p
[46][47][50,51]. These possible effects of miRNA on insulin sensitivity may have a significant role in enhancing symptoms related to PCOS by increasing glucose metabolism and transport
[48][52]. Furthermore, miR-143-3p and miR-155-5p have been shown to antagonize glycolysis in patients with PCOS-related follicular dysplasia. A study by Cao et al. demonstrated that follicular granulosa cells in patients with PCOS had high testosterone as well as a glucose-enriched environment. MiR-143-3p has been demonstrated to negatively regulate glycolysis, while miR-155-5p positively regulates glycolysis
[49][53]. This provides two potential options that require further study as a treatment target in PCOS patients.
3.3. Cardiovascular Complications
There is conflicting evidence on the relationship between PCOS and cardiovascular disease (CVD)
[50][51][55,56]. However, several studies have suggested that women with PCOS are at a higher risk of developing CVD due to the increased prevalence of obesity and insulin resistance in this population, leading to cardiometabolic dysfunction and an unfavorable metabolic profile
[35][52][38,57]. This risk appears to be greater in women who present with hyperandrogenism
[35][50][38,55]. In addition, subclinical CVD markers, such as endothelial dysfunction, coronary artery calcium scores, carotid intima-media thickness, and other markers, were more likely to be elevated in women with PCOS, indicating a possible risk for future cardiovascular events
[52][53][57,58]. The increased oxidative stress associated with PCOS further increases this risk
[54][59]. Nonetheless, the underlying pathogenesis of CVD in PCOS is still unclear, and several hypotheses have been proposed to explain it.
The role of miRNAs in the pathogenesis of CVD has been well established
[55][60]. However, specific miRNAs associated with CVD in PCOS have not been as extensively described. MiRNA-339-5p was studied for its role in regulating endothelial progenitor cells (EPCs) in PCOS women
[56][61]. EPCs are important in maintaining endothelial function, integrity, and neovascularization, as mature endothelial cells have a limited regenerative capacity
[57][62]. EPC dysfunction is associated with endothelial dysfunction and is a common finding in PCOS patients that increases their risk for developing CVDs
[56][57][61,62]. In the study, the upregulation of miRNA-339-5p was found to inhibit the migration, proliferation, and tubular formation of EPCs by inhibiting silent information regulator 1/peroxisome proliferator-activated receptor gamma coactivator 1-alpha (SIRT1/PGC-1α) and PI3K/AKT signaling pathways, making PCOS women more prone to developing CVD
[56][61]. Findings were more evident in obese PCOS women included in the study
[56][61]. Moreover, the study suggested the use of miRNA-339-5p as a promising potential therapeutic target to improve vascular endothelial health and prognosis in PCOS
[56][61].
Further research needs to be done to fully understand the pathogenesis of CVD in PCOS in relation to miRNAs. However, it is thought that the reduction in vascular endothelial health is the primary driver of CVD in PCOS. MiRNAs can reduce the regenerative capacity of endothelial progenitor cells as well as worsen the metabolic profile of PCOS patients. This contributes to vascular damage and leads to a greater risk of developing CVD.