Bovine leptospirosis is an important disease that affects the reproductive sphere. Due to its high relevance for the bovine production chain in a worldwide scenario, a better understanding of the disease is crucial to reduce its negative impacts. The main agents are strains from the Sejroe serogroup, such as Hardjo and Guaricura, which lead to renal and genital infection. The genital colonization causes a chronic, silent, and subclinical reproductive syndrome, called Bovine Genital Leptospirosis (BGL). Embryonic death, estrus repetition, subfertility, and abortions are the main signs of BGL condition in females. However, although leptospires have been identified in semen, the manifestation of BGL in bulls remains to be clarified.
1. Agents and Epidemiological Aspects
Bovine leptospirosis is a worldwide disease caused by several strains of the spirochete bacteria
Leptospira spp.
[1][8]. Leptospires can be classified by genetic or phenotypic features
[2][9]. In its genetic classification, the
Leptospira genus is divided into approximately 68 species, which are classified as pathogenic (subclade P1), intermediate (subclade P2), and saprophytic (subclades S1 and S2)
[3][10]. Regarding the phenotypic classification, also known as serological, the leptospiral strains are divided based on the characteristics of the lipopolysaccharides (LPS) of their outer membrane
[4][11]. In this serological division, the strains are classified into approximately 300 serovars, which are grouped into 24 serogroups according to the homogeny of their LPS
[5][12].
The main known agents of bovine leptospirosis are the strains of Sejroe serogroup, which are considered adapted to the bovine species and do not require other reservoirs for their maintenance, being transmitted from cow to cow
[2][6][9,13]. Sejroe strains have been also isolated worldwide, besides being frequently demonstrated in serosurveys, and in molecular studies
[7][8][9][14,15,16]. Additionally, this serogroup has been associated with reproductive failures
[10][11][3,17], which means that Sejroe strains play a key role in the reproductive syndrome of bovine genital leptospirosis (BGL). Despite its adaptation to bovine, the importance of these strains can be attributed to the preference of Sejroe for colonizing the uterus
[12][18]. The main serovars of this group are Hardjoprajitno (
L. interrogans) and Hardjobovis (
L. borgpetersenii)
[7][14]. Additionally, serovar Guaricura (
L. santarosai), also belonging to the Sejroe serogroup, has been frequently identified in South American cattle
[11][13][17,19]. However, the dynamics of genital infection, adaptability to the host and the real effects of infection in cattle by serovar Guaricura are still not completely understood. Other agents of bovine leptospirosis are the incidental strains Icterohaemorrhagiae, Pomona, Australis, and Grippotyphosa (
L. interrogans), which are adapted to other hosts but still can infect cattle
[1][14][8,20]. Although incidental infection in bovine is less frequent than Sejroe infection, it can lead to acute manifestation, especially in calves, and abortion outbreaks
[2][9].
The transmission of leptospires, regardless of its host origin, usually occurs by shedding bacteria in the urine and other fluids of the infected animal
[8][14][15,20], and the susceptible bovine will be infected by its contact with the contaminated environment. Sejroe strains are transmitted from cattle to cattle, probably not only by a contaminated environment but also during mating. Sexual transmission in cattle has been widely suggested, occurring in male to female direction and vice versa
[6][15][16][13,21,22] since the presence of pathogenic leptospires has been reported both in male and female reproductive tract
[11][17][18][17,23,24].
2. Pathogenesis of Leptospiral Infection in Bovine
The clinical aspects of bovine leptospirosis indicate that it is an infectious disease involving the reproductive system. Paradoxically, most of the research carried out in cattle focuses on the detection of renal carriers, while genital carriers of leptospires are neglected. For a long time, infection in the reproductive tract was considered to be secondary to renal pathogenesis in chronic infection, and the kidneys were considered the main immunologically privileged site
[19][25]. However, the presence of leptospires in ovaries, ovarian follicles, and oviducts has already been demonstrated
[20][21][26,27], as well as in the uterus
[11][17], cervicovaginal mucus
[18][24], and semen
[17][23], which confirmed the genital tract as an important extra-renal location of leptospires. In addition, a relevant immune response in the reproductive tract concomitant with low serum antibody titers has already been recognized
[22][23][28,29]. Additionally, experimental infection demonstrated long-term colonization of the genital tract by Sejroe strains
[24][30]. Although little is known about the pathogenesis of leptospiral strains in those reproductive sites, it is suggested that reproductive failures would be a direct effect of this genital infection and long-term colonization. Moreover, sexual transmission both in the male-female direction and in the opposite direction has been suggested
[2][6][17][25][9,13,23,31], and uterine infection has been experimentally demonstrated
[24][30]. Still in this context, recent studies show that leptospires of the Sejroe serogroup, adapted to cattle, preferentially colonize the reproductive tract, which seems to be their main colonization site
[12][26][18,32]. In a recent review, this evidence was compiled, and the “Bovine Genital Leptospirosis” (BGL) syndrome was suggested as a disease dissociated from renal infection, rather than a secondary infection
[6][13]. After that, the study of genital infection (even in other animal species) was performed by ours and other groups to confirm its agents, understand pathogenesis, and improve diagnosis methods and control protocols
[27][28][29][30][33,34,35,36].
2.1. Pathogenesis and Clinical Manifestation in Females
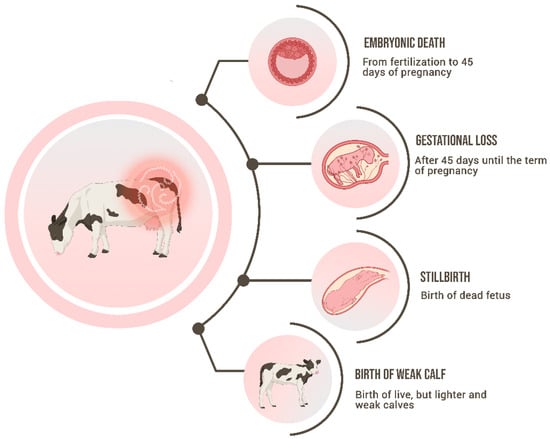
The main signs of BGL are pregnancy losses, which range from embryonic death (more frequently) to late-term abortions (
Figure 1). The embryonic mortality is classified as early embryonic death (EED) when it occurs until 28 days after fertilization, while the late embryonic death (LED) includes the period of 29 to 45 days
[31][37]. In addition, there are pivotal periods in the initial pregnancy trimester
[32][38] that can lead to the death of the embryo/conceptus: (a) Pivotal Period 1, leading to failure in fertilization in the first week after breeding; (b) Pivotal Period 2, which occurs between day 8 to 27, leading to error in the maternal recognition of pregnancy; (c) Pivotal Period 3, which is characterized by the placentome development between days 28 and 60 after breeding.
Figure 1.
Schematic representation of the main signs of Bovine Genital Leptospirosis.
Regardless of the diagnostic method, it is quite difficult to identify the embryonic death in the field, as well as the exact moment that it happened, especially in the pivotal periods 1 and 2. The cows that suffered EED until day 16 after breeding will return to their cycle regularly, while the cows that suffered losses after this period might be related to irregular return to estrus
[31][32][37,38]. Due to the challenging diagnosis of those pregnancy losses, the precise pathogenesis of how leptospires lead to embryonic death is still unclear. Loureiro and Lilenbaum
[6][13] hypothesized two mechanisms, that can occur either separately or combined: (a) the direct infection of the embryo, leading to its non-viability. In this case, embryo death could occur in Pivotal Period 1 because of the direct damage caused by leptospiral infection. The embryo infection by leptospires had been already demonstrated by the penetration of zona pellucida in an in vitro study
[18][24] and was later reinforced by the presence of leptospiral DNA in the follicular fluid of naturally infected cows
[21][27]; or (b) the inflammation of the endometrium caused by leptospiral infection
[33][39] impairs the uterine environment and the embryonic development, being related to the Pivotal Period 2.
Although the presence of leptospires in the uterus of naturally infected cows has been reported
[11][16][17,22], the exact effect of the infection on embryo development remains to be elucidated. In the physiology of pregnancy, the integrity of the endometrium and its function are pivotal to the nourishment and attachment of the embryo
[34][40]. In that context, the oviducts and endometrium secrete a complex fluid, termed histotrophs, that nourishes the conceptus, being its’ only source of nutrients until implantation, usually on the 20th day after fertilization in cows
[35][41]. Previous studies demonstrated that disturbances of the histotroph composition or production can impair pregnancy establishment
[34][40], and other bacterial infections, such as
Escherichia coli or
Trueperella pyogenes in the endometrium can alter the composition of these important secretions
[36][42]. In that context, although the uterine immune response to the infection by
Leptospira spp. is still unclear, researchers hypothesize that one of its effects could be the dysregulation of the histotroph composition or even the reduction or compromising of its secretion. That hypothesis supports the role of the alteration of the uterine environment leading to embryonic death, especially in early gestation.
Since BGL is a chronic infection, subclinical signs can remain for years causing long-term subfertility, and therefore, leading to extensive economic hazards in the herd. In that context, the cow will be classified as a repeat breeder due to frequent embryonic death, presenting subfertility, or even infertility. A direct effect of embryonic mortality is the reduction in the conception rates, which certainly will impair the profitability of the herd
[31][37][37,43]. Other signs that are a consequence of leptospiral infection but occur less frequently than the subclinical signs are abortions, stillbirths, or the birth of weak calves. Abortions have been widely associated with bovine leptospirosis, perhaps the most visible sign, being linked to incidental strains when occur as an outbreak, or to adapted strains when occur endemically
[38][44]. It was suggested that abortions by leptospiral infection occur in the final third of pregnancy; however, fetal death has been reported in all stages
[39][45]. The pathogeny of fetal death is also unclear, but the direct infection of the fetuses is the most probable cause. The lesions found in aborted fetuses positive to leptospires are widely variable, affecting different organs at different levels. The abortions infected by incidental strains such as Pomona or Icterohaemorrhagiae usually present icteric and leptospires may be demonstrated in the kidneys and liver
[40][46]. In contrast, anicteric fetuses with hemorrhagic or even presenting no visible lesions are often associated with infection by Sejroe, which may be demonstrated in several organs, such as cardiopulmonary tissue, thymus, subcapsular kidney content, and abomasal liquid
[39][45].
Besides abortions, stillbirths and the birth of weak and lighter calves also occur as a consequence of bovine leptospirosis
[2][9]. In the case of leptospirosis in horses, the birth of weak foals is associated with placentitis
[41][47]. The inflammation of the bovine placenta has not yet been associated with genital infection by leptospires, and further studies should be performed to understand if placentitis could play a role in bovine abortion pathogenesis. The birth of a dead fetus before or during calving at full term is defined as stillbirth
[42][48]. The exact mechanism of how it happens has not been fully understood. All those clinical signs have the same pathogenesis as the abortions, being caused by the fetal intrauterine infection, but in this case, in late gestation
[2][9].
2.2. Aspects of Genital Infection in Males
Males can also carry leptospires in their reproductive tract. Genital infection has been identified in bulls
[15][17][21,23], semen of rams
[27][33] bucks
[43][49], boars
[44][50], and camelids
[45][51]. Nevertheless, the effect of genital infection by leptospires in males is still poorly understood, and consequently, leptospiral infection in bulls has been highly neglected. In a recent systematic review about the role of bulls’ infectious infertility, leptospirosis has not even been mentioned
[46][52], reflecting its underestimation. Conversely, in a recent critical review, leptospirosis was classified as a potentially transmitted disease of bulls
[47][53], albeit it was less emphasized than other infectious diseases such as trichomoniasis and campylobacteriosis. Although the presence of leptospires in semen seems clear, the real effects of this infection on the semen quality and fertility of the bulls have not yet been clarified. One single study enlightened this issue, showing a correlation between the infection of naturally infected bulls seroreactive against Sejroe with the presence of necrospermia or azoospermia
[48][54]. However, although there is some evidence that leptospiral infection can impair semen quality, more research should be performed in this field. If the infection indeed jeopardizes the semen quality, a new concern is added to the presence of BGL in bulls, besides their crucial role in the epidemiology of the syndrome.
 Encyclopedia
Encyclopedia
