Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Jeong Hee Hong.
Bicarbonate transporters are responsible for the appropriate flux of bicarbonate across the plasma membrane to perform various fundamental cellular functions. The functions of bicarbonate transporters, including pH regulation, cell migration, and inflammation, are highlighted in various cellular systems, encompassing their participation in both physiological and pathological processes.
- bicarbonate transporter
- cellular homeostasis
- channelopathy
- inflammation
1. Homeostatic Role of Bicarbonate Transporters
Bicarbonate is a fundamental ion in cellular pH regulation and crosses the membrane via various bicarbonate transporters, such as the chloride/bicarbonate exchangers (CBE; SLC26A family and anion exchanger (AE) family), sodium/bicarbonate cotransporter (NBC), sodium-dependent chloride–bicarbonate exchanger (NDCBE), and the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) channel [1,2,3,4,5][1][2][3][4][5]. It has been known that the human adult bicarbonate level is maintained between 23 to 29 mEq/L in serum and 10 mEq/L in the cytosol [6]. Since bicarbonate transporters were first identified, their biological and physiological roles have been revealed in various cellular systems, including pathological processes. Defects in bicarbonate transporters result in disease-state signaling [7].
2. Inflammatory Disease-Associated Cytokines
Inflammatory cytokines increase bicarbonate secretion in epithelia [12,13][8][9]. The pH of airway epithelial surface liquid, for example, is a critical factor in respiratory defense against inhaled pathogens [14][10]. Alkalization of the cell surface fluid through bicarbonate secretion is associated with host defense mechanisms. Thus, the roles of bicarbonate transporters in inflammatory settings have been investigated in several studies of airway and intestinal systems, and these findings are discussed in this section. Among the chloride–bicarbonate exchangers, downregulated in adenoma (DRA, also termed SLC26A3) is found in the gastrointestinal system and is mainly implicated in inflammatory diseases. For instance, the interleukin (IL)-10-deleted colitis mouse model and patients with ulcerative colitis revealed reduced DRA expression [15][11]. The downregulation of DRA leads to a defect in bicarbonate secretion and may affect the protective function against overt diarrhea [16][12]. In addition, inflammatory diseases, such as bowel disease, reveal the enhanced tumor necrosis factor (TNF)-α levels in the mucosa and lamina propria [17,18][13][14]. Several studies have verified the relationship between bicarbonate transporters and TNF-α. Treatment with TNF-α reduces mRNA and protein expression of DRA in colorectal adenocarcinoma HT-29 cells and Caco-2 3D-cysts model [19][15]. Moreover, injection of TNF-α decreases mRNA and protein expression levels of DRA compared to controls in crypt-derived mouse ileal enteroids on matrigel [19][15]. Overexpression of nuclear factor-kappa B (NF-κB) subunits also inhibits mRNA and protein expression levels of DRA [19][15]. Conversely, mRNA expression of DRA is increased by the treatment of NF-κB inhibitor caffeic acid phenethyl ester in HT-29 cells and ileal enteroids [19][15]. In ileal enteroid-derived mouse crypts, TNF-α decreases DRA mRNA expression, while having no effect on another CBE, Slc26a6 (mouse PAT-1) [19][15]. DRA knockout mice, not PAT-1 knockout, reveal dysregulated electrolyte balance; thus DRA, not PAT-1, is important in the regulation of intestinal chloride and fluid volume [20][16]. In ulcerative colitis (UC) patient samples, DRA expression levels are decreased, whereas serum TNF-α levels are upregulated compared to a control group [21][17]. Consistent with these findings, in a dextran sulfate sodium (DSS)-induced colitis mouse model, expression levels of DRA mRNA and protein are downregulated, whereas TNF-α is upregulated [21][17]. In DRA-overexpressed Caco2-brush border of enterocyte (Caco2/BBE) cells, TNF-α treatment induces the downregulation of DRA [21][17]. Knockdown of TNF-α with siRNA significantly upregulates mRNA and protein expression of DRA [21][17]. Conversely, knockdown of DRA with siRNA induces the upregulated mRNA and protein expression of TNF-α compared to control [21][17]. Thus, stability of DRA might be affected by inflammatory cytokines and DRA activity is dominantly involved in the maintenance of electrolyte homeostasis. Additionally, it is known that anion exchanger 2 (AE2) secretes bicarbonate to protect biliary mucosa in the digestive system from related diseases such as primary biliary cholangitis (PBC) [22][18]. Pro-inflammatory cytokines such as IL-8, IL-12, IL-17, IL-18, and TNF-α may contribute to the reduced expression and activity of AE2 by enhancing miR-506 expression in H69 cholangiocytes [23][19]. Dysregulated AE2 may impair the bicarbonate umbrella against bile salt-associated cellular apoptosis [23,24][19][20]. In addition to DRA and AE2, pendrin, also termed SLC26A4, has been associated with the airway system. IL-17 treatment time-dependently enhances mRNA and protein expression of pendrin in human bronchial epithelial (HBE) cells [25][21]. DRA mRNA is decreased with IL-17 treatment in HBE cells [25][21]. Regarding DRA expression in tissues, DRA is not detected in the respiratory system [26][22]. In the presence of the NF-κB inhibitor JSH-23, IL-17 treatment does not increase pendrin mRNA and protein expression in HBE cells [25][21]. In cystic fibrosis (CF) HBE cells, pendrin mRNA expression and pHi are increased, whereas treatment with siRNA-pendrin decreases chloride/bicarbonate exchanger activity [25][21]. The combined administration of TNF-α and IL-17 time-dependently elevates the pH of the airway epithelial surface liquid [14][10]. Theoretically, the pH of airway epithelial liquid may increase as a consequence of increased bicarbonate secretion, decreased H+ secretion, or both [14][10]. These results indicate that TNF-α acidifies the pH of airway epithelial liquid by enhancement of H+ secretion, whereas IL-17 has no effect on H+ transport. Additionally, co-treatment with both TNF-α and IL-17 increases the pH of airway epithelial liquid through enhanced bicarbonate secretion, not by reduced H+ secretion, in human airway epithelia [14][10]. The combined treatment of IL-17 and TNF-α increases the mRNA expression of a gene subset of bicarbonate transporters, including CFTR, members of the SLC26A family, and the SLC4 family (NBCs) and its associated enzymes, carbonic anhydrases (CAs), in human airway epithelia [14][10]. WThe researchers included CFTR in this reviewsearch because the chloride channel CFTR is considered as the bicarbonate efflux transporter [27,28][23][24]. Concomitant addition of IL-17 and TNF-α with CFTR (inh)-172, a CFTR inhibitor, induces decreased pH of airway epithelial liquid [29][25]. Co-treatment of IL-17 and TNF-α with CFTR modulators (VX-445, VX-661, and VX-770) causes an increase in the pH of airway epithelial surface liquid [29][25]. Additionally, treatment with pendrin siRNA in the co-presence of IL-17 and TNF-α induces reduced pH of airway epithelial liquid [14][10]. Thus, combined treatment with IL-17 and TNF-α induces bicarbonate secretion through the involvement of CFTR and pendrin [29][25], alkalinizing the pH of airway epithelial liquid and providing an important barrier defense mechanism against airway inflammation (Figure 1). Understanding the effects of cytokines on the pH regulation of airway surface fluid through bicarbonate transporters would support the development of therapeutic strategies against airway inflammation.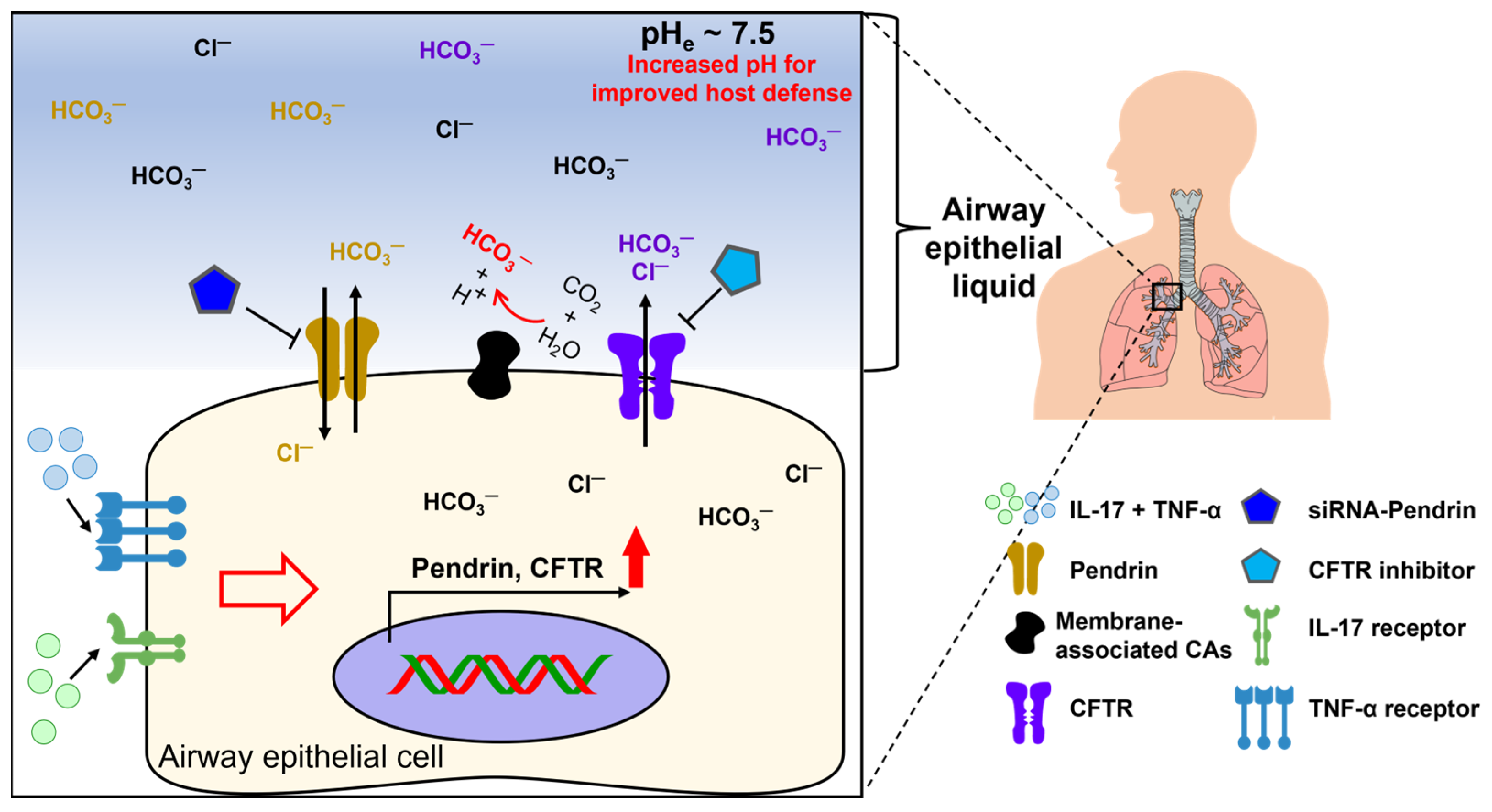
Figure 1. Schematic illustration of inflammatory cytokine-mediated ion transporters in airway epithelia. Bicarbonate secretion by airway epithelial transporters plays a crucial role in the barrier defense mechanism against inflammation by enhancing the pH of airway epithelial fluid. The presence of TNF and IL-17 in airway epithelial fluid enhances the efficacy of CFTR and pendrin function [29][25]. CFTR: cystic fibrosis transmembrane conductance regulator, CAs: carbonic anhydrases (enhanced membrane-associated CA9 and CA12) [14][10].
3. Angiotensin II-Mediated Modulation of Bicarbonate Transport
Angiotensin II (ANG II) regulates systemic and renal circulation through the release of aldosterone in the adrenal cortex. It is known that ANG II is involved in bicarbonate reabsorption in the proximal tubule [30][26]. ANG II-mediated bicarbonate absorption occurs through the involvement of the bicarbonate transporter NBC in proximal tubules [30,31,32][26][27][28]. ANG II-mediated intracellular pH modulation is also involved in AE activity. ANG II treatment facilitates the reduction of myocardial intracellular pH by activating AE under conditions of an alkali load induced by trimethylamine hydrochloride (TMA), a protein kinase C (PKC) activator, treatment in cat papillary muscles [43][29]. Among the AE family members AE1 to AE3, full-length AE3 is involved in ANG II-mediated activation of PKC in the myocardium [44][30]. Additionally, the protein expression of AE1 is upregulated by a combination of the aldosterone analogue deoxycorticosterone acetate and NaHCO3 to induce alkalosis in the mouse kidney medulla, helping to maintain acid–base balance [45][31]. In addition, although sodium-chloride cotransporter (NCC) is not included in the bicarbonate transporter family, the NCC in plasma membrane is enhanced by ANG II treatment, and co-stimulation of aldosterone enhances pendrin expression, along with the expression of phosphorylated NCC, in adrenalectomized mice [46][32]. This implies that the upregulation of NCCs and pendrin by ANG II is required for the maintenance of blood pressure via the renin–ANG–aldosterone system. Furthermore, treatment with ANG II decreases CFTR mRNA and protein expression in the basilar arteries of the brain [47][33]. The Weresearchers illustrated the differential role of ANG II in Figure 2. WThe researchers emphasized that CFTR is understood to be a regulator of vasoconstriction. Thus, the effect of ANG II on the modulation of bicarbonate transporters is complex and requires careful consideration, taking into account the different functions of bicarbonate transporters such as pendrin and CFTR in each organ.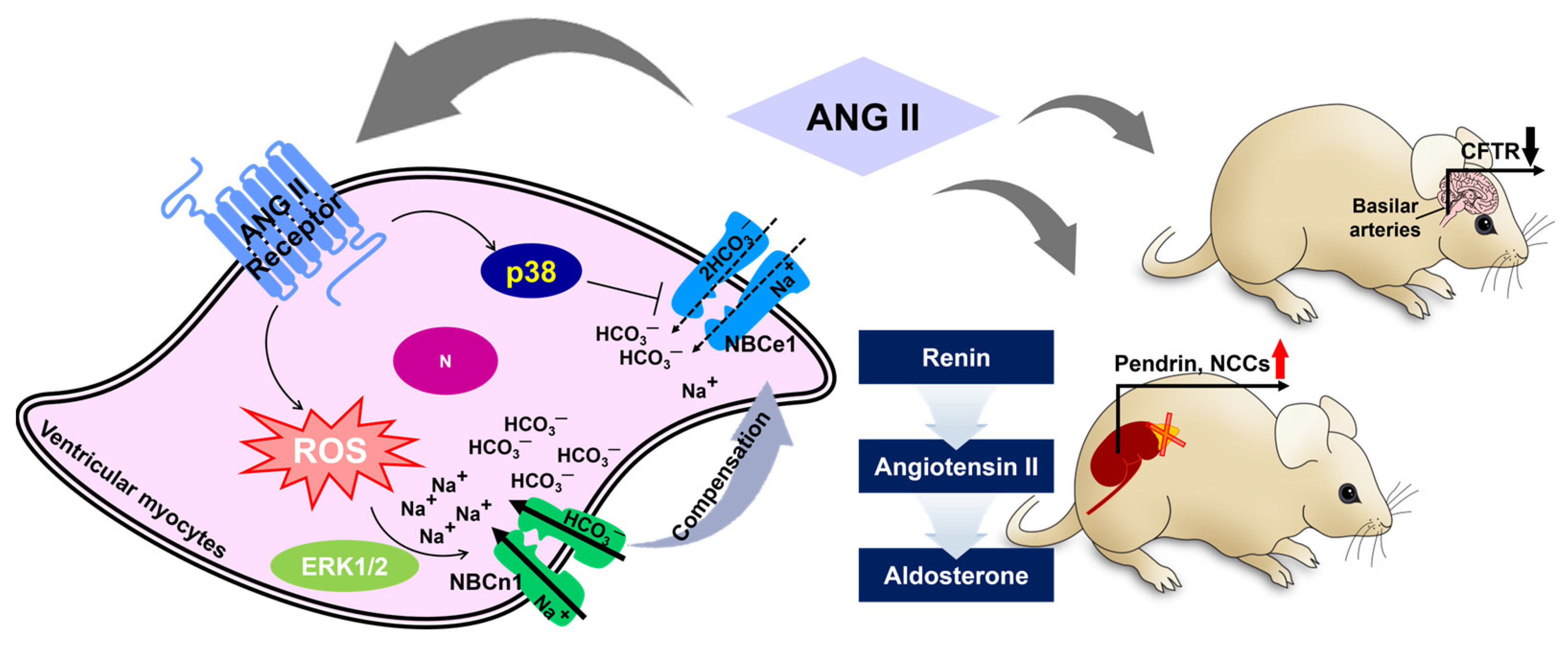
Figure 2. Schematic illustration of angiotensin II-mediated bicarbonate transporter regulation. ANG II represents differential roles in NBCs in ventricular myocytes [40,41][34][35]. There are differential effects of ANG II on pendrin/NCC and CFTR in the kidney and basilar arteries of the brain, respectively [46,47][32][33]. Black and red arrow indicates down- or up-regulated protein expression, respectively. ANG II: angiotensin II, ERK1/2: extracellular signal-regulated kinase1/2, ROS: reactive oxygen species, N: nucleus, NCCs: sodium chloride cotransporter, CFTR: cystic fibrosis transmembrane conductance regulator.
4. Regulation of Bicarbonate Transport by Endogenous Peptides
4.1. Neuropeptide Vasoactive Intestinal Peptide
Vasoactive intestinal polypeptide (VIP) is a 28-amino-acid neuropeptide produced by neurons, T cells, B cells, and endocrine cells and is found in most organs [48,49][36][37]. VIP is released in response to various stimuli, including serotonin, acetylcholine, substance P, and others [49][37]. VIP stimulation contributes to the regulation of inflammation and smooth muscle relaxation [50][38]. It also has been reported that VIP activates bicarbonate transport by involving CFTR in mice with disrupted CFTR genes, established as a colony model for cystic fibrosis [51][39]. VIP is a physiological activator of CFTR, contributing to mucus hydration and local nonspecific immune system activity in mammalian airways [52,53][40][41]. VIP enhances CFTR activity through both protein kinase A (PKA)- and PKC-dependent molecular mechanisms [54,55,56][42][43][44]. Stimulation of VIP receptors (VPAC1 and VPAC2) enhances cAMP and calcium production through the G protein-coupled VPACs and subsequently activates CFTR function [54,55,57][42][43][45]. Accordingly, VIP is a regulator of CFTR-mediated secretion in respiratory submucosal glands [52,58][40][46]. In Calu-3 lung epithelial cells, treatment with VIP time-dependently increases CFTR expression in the apical membrane [59][47]. VIP-induced CFTR expression is downregulated via the treatment of PKC inhibitors bisindolylmaleimide X or chelerythrine chloride [59][47]. Oxidative damage caused by ozone exposure or cigarette smoke leads to an upregulation of glutathione and a downregulation of chloride secretion by reducing CFTR expression levels at the apical side in Calu-3 and T84 cells [60,61][48][49]. Ozone stress induces the downregulation of CFTR expression and activity through the signal transducer and activator of transcription 1 (STAT1) signal pathway in HBE cells [62][50]. VIP treatment has been shown to recover ozone stress-mediated CFTR dysfunction in HBE cells through the PKA and PKC signaling pathway [56][44]. Furthermore, treatment with the PKCε inhibitor peptide, Epsilon-V1-2, reduces the surface expression of CFTR in the presence of VIP in Calu-3 cells [63][51], suggesting that PKCε is involved in VIP-modulated CFTR membrane stability. Sodium–hydrogen exchange regulatory factor-1 (NHERF1) is a PDZ domain-containing scaffolding protein that interacts with CFTR [64,65][52][53]. VIP exposure has been shown to enhance NHERF1 expression and co-localization with CFTR [63][51]. However, VIP treatment in the presence of siRNA-NHERF1 does not enhance the surface expression of CFTR compared to VIP treatment alone [63][51]. These results suggest that NHERF1 participates in the VIP-CFTR signaling cascade. Although VIP-mediated bicarbonate or chloride regulation is well-defined for CFTR function [66[54][55][56][57],67,68,69], the verification of regulatory interactions between VIP and other bicarbonate transporters should be investigated in the future.4.2. Neuropeptide Y
Neuropeptide Y (NPY) is a 36-amino acid peptide hormone extensively expressed in submucosal neurons and the myenteric plexus throughout the gastrointestinal tract of humans [70][58], mice [71][59], and rats [72][60]. The NPY family includes NPY, peptide YY, and pancreatic polypeptide [73][61] (sequences are illustrated in Figure 3), sharing significant biological homology [74,75,76,77][62][63][64][65]. Among them, NPY is known to be a vasoconstriction peptide [78][66]. Since NPY is secreted simultaneously with norepinephrine (NE) in sympathetic responses, NE-induced vasoconstriction is accelerated by NPY-mediated NPY Y1 receptor activation [78][66].
5. Intracellular Calcium-Associated Modulation of Bicarbonate Transport
Intracellular calcium homeostasis is a critical process for maintaining various physiological functions, including cardiac and neural function [85[70][71],86], and is regulated by numerous signaling proteins in a highly coordinated manner. In addition, drugs that modulate calcium signaling play a role in the regulation of bicarbonate transporters. Treatment with the calcium ionophore 4Br-A23187 increases intracellular calcium, while it inhibits DRA activity in DRA-transfected human embryonic kidney (HEK) cells [89][72]. Regarding calcium signaling-associated proteins, as a calcium-associated protein, IRBIT (IP3R coupling protein released with inositol 1,4,5-trisphosphate) has been identified as a molecule that competes with IP3 for binding to the IP3 receptor [90,91][73][74] and is now recognized as a multifactorial regulator due to its wide range of target proteins such as NBCe1-B [92][75] and calcium/calmodulin kinase IIα [93][76]. IRBIT binds to NBCe1-B, one variant of NBCe1, and regulates NBC activity, playing a critical role in epithelial secretion [92,94][75][77]. The activity of NBCe1-B exhibits basal properties due to the auto-inhibitory domain (AID), which consists of residues 1-85 [95,96][78][79]. The AID of NBCe1-B is released by the binding of IRBIT through electrostatic interaction, resulting in the activation of NBCe1-B [96,97,98][79][80][81]. In addition, AE2 is identified as one of the binding targets of both IRBIT and long (L)-IRBIT in HEK293T cells [99][82]. L-IRBIT KO via CRISPR/Cas9 induces downregulated AE2 activity and expression in B16F10 melanoma cells [99][82]. IRBIT/L-IRBIT double KO reduces AE2 expression, whereas IRBIT overexpression restores AE2 activity in IRBIT/L-IRBIT double KO B16-F10 cells [99][82]. In L-IRBIT KO B16F10 cells, the lysosomal degradation inhibitor bafilomycin upregulates AE2 protein expression and recovers L-IRBIT KO-induced AE2 downregulation [99][82]. IRBIT and L-IRBIT possess the AHCY domain, which forms multimer. The AE2 binds to IRBIT multimer, which contains homo-multimer or hetero-multimer in HEK293T cells [99][82]. Although only IRBIT homo-multimer is involved in the regulation of AE2 expression, the binding of IRBIT family modulates the protein stability of AE2 through the modulation of endosome/lysosome-dependent degradation pathway. Additionally, IRBIT antagonizes kinases such as Ste20-related proline/alanine-rich kinase (SPAK) or with-no-lysine (WNK) kinase, which destabilizes membrane expression of NBCe1-B [92][75]. Hwang et al. reported that IRBIT stabilizes NBCn1 expression in the plasma membrane to support cellular migration in A549 lung cancer cells [100][83]. Knockdown of IRBIT destabilizes plasma NBCn1 expression and reduces cellular migration, suggesting that IRBIT mediates the maintenance of a stable migratory module [100][83]. Although the calcium-associated protein IRBIT is related to several bicarbonate transporters such as AE2 and NBCs, identifying the regulatory relationships between IRBIT or IRBIT family and other bicarbonate transporters will be challenging in the future.References
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S24–S30.
- Boron, W.F.; Fong, P.; Hediger, M.A.; Boulpaep, E.L.; Romero, M.F. The electrogenic Na/HCO3 cotransporter. Wien. Klin. Wochenschr. 1997, 109, 445–456.
- Kopito, R.R. Molecular biology of the anion exchanger gene family. Int. Rev. Cytol. 1990, 123, 177–199.
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Aspects Med. 2013, 34, 494–515.
- Yang, O.C.Y.; Loh, S.H. Acidic Stress Triggers Sodium-Coupled Bicarbonate Transport and Promotes Survival in A375 Human Melanoma Cells. Sci. Rep. 2019, 9, 6858.
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011; 1091p.
- Cordat, E.; Casey, J.R. Bicarbonate transport in cell physiology and disease. Biochem. J. 2009, 417, 423–439.
- Kreindler, J.L.; Bertrand, C.A.; Lee, R.J.; Karasic, T.; Aujla, S.; Pilewski, J.M.; Frizzell, R.A.; Kolls, J.K. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L257–L266.
- Gorrieri, G.; Scudieri, P.; Caci, E.; Schiavon, M.; Tomati, V.; Sirci, F.; Napolitano, F.; Carrella, D.; Gianotti, A.; Musante, I.; et al. Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release. Sci. Rep. 2016, 6, 36016.
- Rehman, T.; Thornell, I.M.; Pezzulo, A.A.; Thurman, A.L.; Ibarra, G.S.R.; Karp, P.H.; Tan, P.; Duffey, M.E.; Welsh, M.J. TNF alpha and IL-17 alkalinize airway surface liquid through CFTR and pendrin. Am. J. Physiol.-Cell Physiol. 2020, 319, C331–C344.
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. 1998, 275, G1445–G1453.
- Xiao, F.; Juric, M.; Li, J.; Riederer, B.; Yeruva, S.; Singh, A.K.; Zheng, L.; Glage, S.; Kollias, G.; Dudeja, P.; et al. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3− secretion in murine ileocolonic inflammation. Inflamm. Bowel Dis. 2012, 18, 101–111.
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006.
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10.
- Kumar, A.; Chatterjee, I.; Gujral, T.; Alakkam, A.; Coffing, H.; Anbazhagan, A.N.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; et al. Activation of Nuclear Factor-κB by Tumor Necrosis Factor in Intestinal Epithelial Cells and Mouse Intestinal Epithelia Reduces Expression of the Chloride Transporter SLC26A3. Gastroenterology 2017, 153, 1338–1350.e3.
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 2006, 281, 37962–37971.
- Ding, X.M.; Li, D.X.; Li, M.K.; Tian, D.; Yu, H.B.; Yu, Q. Tumor necrosis factor-α acts reciprocally with solute carrier family 26, member 3, (downregulated-in-adenoma) and reduces its expression, leading to intestinal inflammation. Int. J. Mol. Med. 2018, 41, 1224–1232.
- Sasaki, M.; Sato, Y.; Nakanuma, Y. An impaired biliary bicarbonate umbrella may be involved in dysregulated autophagy in primary biliary cholangitis. Lab. Investig. 2018, 98, 745–754.
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Aguero, R.; Fernandez-Irigoyen, J.; et al. MicroRNA-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology 2018, 67, 1420–1440.
- Chang, J.C.; Go, S.; de Waart, D.R.; Munoz-Garrido, P.; Beuers, U.; Paulusma, C.C.; Oude Elferink, R. Soluble Adenylyl Cyclase Regulates Bile Salt-Induced Apoptosis in Human Cholangiocytes. Hepatology 2016, 64, 522–534.
- Adams, K.M.; Abraham, V.; Spielman, D.; Kolls, J.K.; Rubenstein, R.C.; Conner, G.E.; Cohen, N.A.; Kreindler, J.L. IL-17A induces Pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS ONE 2014, 9, e103263.
- Wheat, V.J.; Shumaker, H.; Burnham, C.; Shull, G.E.; Yankaskas, J.R.; Soleimani, M. CFTR induces the expression of DRA along with Cl−/HCO3− exchange activity in tracheal epithelial cells. Am. J. Physiol. Cell Physiol. 2000, 279, C62–C71.
- Poulsen, J.H.; Fischer, H.; Illek, B.; Machen, T.E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA 1994, 91, 5340–5344.
- Garnett, J.P.; Hickman, E.; Burrows, R.; Hegyi, P.; Tiszlavicz, L.; Cuthbert, A.W.; Fong, P.; Gray, M.A. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J. Biol. Chem. 2011, 286, 41069–41082.
- Rehman, T.; Karp, P.H.; Tan, P.; Goodell, B.J.; Pezzulo, A.A.; Thurman, A.L.; Thornell, I.M.; Durfey, S.L.; Duffey, M.E.; Stoltz, D.A.; et al. Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators. J. Clin. Investig. 2021, 131, e150398.
- Geibel, J.; Giebisch, G.; Boron, W.F. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc. Natl. Acad. Sci. USA 1990, 87, 7917–7920.
- Romero, M.F.; Hopfer, U.; Madhun, Z.T.; Zhou, W.; Douglas, J.G. Angiotensin II actions in the rabbit proximal tubule. Angiotensin II mediated signaling mechanisms and electrolyte transport in the rabbit proximal tubule. Ren. Physiol. Biochem. 1991, 14, 199–207.
- Coppola, S.; Fromter, E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. I. Effect of picomolar concentrations. Pflugers Arch. 1994, 427, 143–150.
- Camilion de Hurtado, M.C.; Alvarez, B.V.; Perez, N.G.; Ennis, I.L.; Cingolani, H.E. Angiotensin II activates Na+-independent Cl−-HCO3− exchange in ventricular myocardium. Circ. Res. 1998, 82, 473–481.
- Alvarez, B.V.; Fujinaga, J.; Casey, J.R. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ. Res. 2001, 89, 1246–1253.
- Mohebbi, N.; Perna, A.; van der Wijst, J.; Becker, H.M.; Capasso, G.; Wagner, C.A. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS ONE 2013, 8, e55286.
- Hirohama, D.; Ayuzawa, N.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Watanabe, A.; Shimosawa, T.; Marumo, T.; Shibata, S.; Fujita, T. Aldosterone Is Essential for Angiotensin II-Induced Upregulation of Pendrin. J. Am. Soc. Nephrol. 2018, 29, 57–68.
- Zhao, L.; Yuan, F.; Pan, N.; Yu, Y.; Yang, H.; Liu, Y.; Wang, R.; Zhang, B.; Wang, G. CFTR deficiency aggravates Ang II induced vasoconstriction and hypertension by regulating Ca2+ influx and RhoA/Rock pathway in VSMCs. Front. Biosci. 2021, 26, 1396–1410.
- Orlowski, A.; Ciancio, M.C.; Caldiz, C.I.; De Giusti, V.C.; Aiello, E.A. Reduced sarcolemmal expression and function of the NBCe1 isoform of the NaHCO3− cotransporter in hypertrophied cardiomyocytes of spontaneously hypertensive rats: Role of the reninangiotensin system. Cardiovasc. Res. 2014, 101, 211–219.
- De Giusti, V.C.; Orlowski, A.; Aiello, E.A. Angiotensin II inhibits the electrogenic Na+/HCO3− cotransport of cat cardiac myocytes. J. Mol. Cell. Cardiol. 2010, 49, 812–818.
- Henning, R.J.; Sawmiller, D.R. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc. Res. 2001, 49, 27–37.
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629.
- Leceta, J.; Gomariz, R.P.; Martinez, C.; Carrion, M.; Arranz, A.; Juarranz, Y. Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. Neuroimmunomodulation 2007, 14, 134–138.
- Hogan, D.L.; Crombie, D.L.; Isenberg, J.I.; Svendsen, P.; Schaffalitzky de Muckadell, O.B.; Ainsworth, M.A. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology 1997, 113, 533–541.
- Wine, J.J.; Joo, N.S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53.
- Maggi, C.A.; Giachetti, A.; Dey, R.D.; Said, S.I. Neuropeptides as Regulators of Airway Function—Vasoactive-Intestinal-Peptide and the Tachykinins. Physiol. Rev. 1995, 75, 277–322.
- Derand, R.; Montoni, A.; Bulteau-Pignoux, L.; Janet, T.; Moreau, B.; Muller, J.M.; Becq, F. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. Br. J. Pharmacol. 2004, 141, 698–708.
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639.
- Qu, F.; Liu, H.J.; Xiang, Y.; Tan, Y.R.; Liu, C.; Zhu, X.L.; Qin, X.Q. Activation of CFTR trafficking and gating by vasoactive intestinal peptide in human bronchial epithelial cells. J. Cell. Biochem. 2011, 112, 902–908.
- Rumalla, K.; Yarbrough, C.K.; Pugely, A.J.; Koester, L.; Dorward, I.G. Spinal fusion for pediatric neuromuscular scoliosis: National trends, complications, and in-hospital outcomes. J. Neurosurg. Spine 2016, 25, 500–508.
- Ianowski, J.P.; Choi, J.Y.; Wine, J.J.; Hanrahan, J.W. Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice. J. Physiol. 2007, 580, 301–314.
- Chappe, F.; Loewen, M.E.; Hanrahan, J.W.; Chappe, V. Vasoactive intestinal peptide increases cystic fibrosis transmembrane conductance regulator levels in the apical membrane of Calu-3 cells through a protein kinase C-dependent mechanism. J. Pharmacol. Exp. Ther. 2008, 327, 226–238.
- Cantin, A.M.; Bilodeau, G.; Ouellet, C.; Liao, J.; Hanrahan, J.W. Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 2006, 290, C262–C270.
- Cantin, A.M.; Hanrahan, J.W.; Bilodeau, G.; Ellis, L.; Dupuis, A.; Liao, J.; Zielenski, J.; Durie, P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care 2006, 173, 1139–1144.
- Qu, F.; Qin, X.Q.; Cui, Y.R.; Xiang, Y.; Tan, Y.R.; Liu, H.J.; Peng, L.H.; Zhou, X.Y.; Liu, C.; Zhu, X.L. Ozone stress down-regulates the expression of cystic fibrosis transmembrane conductance regulator in human bronchial epithelial cells. Chem. Biol. Interact. 2009, 179, 219–226.
- Alshafie, W.; Chappe, F.G.; Li, M.S.; Anini, Y.; Chappe, V.M. VIP regulates CFTR membrane expression and function in Calu-3 cells by increasing its interaction with NHERF1 and P-ERM in a VPAC1-and PKC ε-dependent manner. Am. J. Physiol.-Cell Physiol. 2014, 307, C107–C119.
- Short, D.B.; Trotter, K.W.; Reczek, D.; Kreda, S.M.; Bretscher, A.; Boucher, R.C.; Stutts, M.J.; Milgram, S.L. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 1998, 273, 19797–19801.
- Cheng, J.; Moyer, B.D.; Milewski, M.; Loffing, J.; Ikeda, M.; Mickle, J.E.; Cutting, G.R.; Li, M.; Stanton, B.A.; Guggino, W.B. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J. Biol. Chem. 2002, 277, 3520–3529.
- Yu, B.; Zhu, X.; Yang, X.; Jin, L.; Xu, J.; Ma, T.; Yang, H. Plumbagin Prevents Secretory Diarrhea by Inhibiting CaCC and CFTR Channel Activities. Front. Pharmacol. 2019, 10, 1181.
- McMahon, D.B.; Carey, R.M.; Kohanski, M.A.; Tong, C.C.L.; Papagiannopoulos, P.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Neuropeptide regulation of secretion and inflammation in human airway gland serous cells. Eur. Respir. J. 2020, 55, 1901386.
- Oak, A.A.; Chhetri, P.D.; Rivera, A.A.; Verkman, A.S.; Cil, O. Repurposing calcium-sensing receptor agonist cinacalcet for treatment of CFTR-mediated secretory diarrheas. JCI Insight 2021, 6, e146823.
- Rodrat, M.; Wongdee, K.; Teerapornpuntakit, J.; Thongbunchoo, J.; Tanramluk, D.; Aeimlapa, R.; Thammayon, N.; Thonapan, N.; Wattano, P.; Charoenphandhu, N. Vasoactive intestinal peptide and cystic fibrosis transmembrane conductance regulator contribute to the transepithelial calcium transport across intestinal epithelium-like Caco-2 monolayer. PLoS ONE 2022, 17, e0277096.
- Nichols, K.; Staines, W.; Krantis, A. Neural sites of the human colon colocalize nitric oxide synthase-related NADPH diaphorase activity and neuropeptide Y. Gastroenterology 1994, 107, 968–975.
- Sandgren, K.; Larsson, L.T.; Ekblad, E. Widespread changes in neurotransmitter expression and number of enteric neurons and interstitial cells of Cajal in lethal spotted mice—An explanation for persisting dysmotility after operation for Hirschsprung’s disease? Dig. Dis. Sci. 2002, 47, 1049–1064.
- Ekblad, E.; Ekman, R.; Hakanson, R.; Sundler, F. Projections of peptide-containing neurons in rat colon. Neuroscience 1988, 27, 655–674.
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274.
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170.
- Floyd, J.C., Jr.; Fajans, S.S.; Pek, S.; Chance, R.E. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent. Prog. Horm. Res. 1976, 33, 519–570.
- Tatemoto, K. Neuropeptide Y: Complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. USA 1982, 79, 5485–5489.
- Tatemoto, K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 1982, 79, 2514–2518.
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1281.
- Fang, S.Y.; Shen, C.L.; Ohyama, M. Presence of neuropeptides in human nasal polyps. Acta Otolaryngol. 1994, 114, 324–328.
- Makinde, T.O.; Steininger, R.; Agrawal, D.K. NPY and NPY receptors in airway structural and inflammatory cells in allergic asthma. Exp. Mol. Pathol. 2013, 94, 45–50.
- Chandrasekharan, B.; Nezami, B.G.; Srinivasan, S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G949–G957.
- Barry, W.H.; Bridge, J.H. Intracellular calcium homeostasis in cardiac myocytes. Circulation 1993, 87, 1806–1815.
- Verkhratsky, A.; Orkand, R.K.; Kettenmann, H. Glial calcium: Homeostasis and signaling function. Physiol. Rev. 1998, 78, 99–141.
- Lamprecht, G.; Hsieh, C.J.; Lissner, S.; Nold, L.; Heil, A.; Gaco, V.; Schafer, J.; Turner, J.R.; Gregor, M. Intestinal Anion Exchanger Down-regulated in Adenoma (DRA) Is Inhibited by Intracellular Calcium. J. Biol. Chem. 2009, 284, 19744–19753.
- Ando, H.; Mizutani, A.; Matsu-ura, T.; Mikoshiba, K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J. Biol. Chem. 2003, 278, 10602–10612.
- Ando, H.; Mizutani, A.; Kiefer, H.; Tsuzurugi, D.; Michikawa, T.; Mikoshiba, K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol. Cell 2006, 22, 795–806.
- Yang, D.; Li, Q.; So, I.; Huang, C.L.; Ando, H.; Mizutani, A.; Seki, G.; Mikoshiba, K.; Thomas, P.J.; Muallem, S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J. Clin. Investig. 2011, 121, 956–965.
- Kawaai, K.; Mizutani, A.; Shoji, H.; Ogawa, N.; Ebisui, E.; Kuroda, Y.; Wakana, S.; Miyakawa, T.; Hisatsune, C.; Mikoshiba, K. IRBIT regulates CaMKII α activity and contributes to catecholamine homeostasis through tyrosine hydroxylase phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, 5515–5520.
- Shirakabe, K.; Priori, G.; Yamada, H.; Ando, H.; Horita, S.; Fujita, T.; Fujimoto, I.; Mizutani, A.; Seki, G.; Mikoshiba, K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc. Natl. Acad. Sci. USA 2006, 103, 9542–9547.
- Lee, S.K.; Boron, W.F.; Parker, M.D. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: Role of IRBIT vs.amino-terminal truncation. Am. J. Physiol. Cell Physiol. 2012, 302, C518–C526.
- Hong, J.H.; Yang, D.; Shcheynikov, N.; Ohana, E.; Shin, D.M.; Muallem, S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc. Natl. Acad. Sci. USA 2013, 110, 4105–4110.
- Su, P.; Wu, H.; Wang, M.; Cai, L.; Liu, Y.; Chen, L.M. IRBIT activates NBCe1-B by releasing the auto-inhibition module from the transmembrane domain. J. Physiol. 2021, 599, 1151–1172.
- Wang, M.; Wu, H.; Liu, Y.; Chen, L.M. Activation of mouse NBCe1-B by Xenopus laevis and mouse IRBITs: Role of the variable Nt appendage of IRBITs. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183240.
- Itoh, R.; Hatano, N.; Murakami, M.; Mitsumori, K.; Kawasaki, S.; Wakagi, T.; Kanzaki, Y.; Kojima, H.; Kawaai, K.; Mikoshiba, K.; et al. Both IRBIT and long-IRBIT bind to and coordinately regulate Cl−/HCO3− exchanger AE2 activity through modulating the lysosomal degradation of AE2. Sci. Rep. 2021, 11, 5990.
- Hwang, S.; Shin, D.M.; Hong, J.H. Protective Role of IRBIT on Sodium Bicarbonate Cotransporter-n1 for Migratory Cancer Cells. Pharmaceutics 2020, 12, 816.
More
