Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sian Cartland.
Peripheral artery disease (PAD) is caused by blocked arteries due to atherosclerosis and/or thrombosis which reduce blood flow to the lower limbs. It results in major morbidity, including ischemic limb, claudication, and amputation, with patients also suffering a heightened risk of heart attack, stroke, and death.
- peripheral artery disease
- endothelial cell dysfunction
- sex differences
1. Introduction
Peripheral artery disease (PAD) is a disease with high human and social impact, significantly reducing the quality of life. In this condition, an impairment of the blood supply due to atherosclerosis results in ischemia, most commonly to the lower limbs. More than 230 million people are affected by PAD globally [1], with the prevalence expected to increase because of a rise in diabetes mellitus [2]. In severe cases, patients, particularly those with diabetes, develop gangrene, which necessitates surgical amputation of the limbs. In the United States alone, 185,000 limbs are amputated every year, and it is estimated that by 2050, >3.5 million U.S. citizens will live without a limb [3]. Although revascularization surgery can improve perfusion, the current interventions may be insufficient because of extensive disease. Furthermore, the underlying atherosclerotic disease remains, and patients with PAD frequently undergo multiple vascular surgical procedures, each of which increases the risk of heart attack and stroke [4]. Indeed, patients who have undergone below- or above-knee amputations are more likely to die within 5 years; a mortality rate greater than those of breast, colon, and prostate cancer [5]. PAD, therefore, causes major trauma and disability, costing the U.S. economy USD 84–380 billion/year [6].
Remarkably, PAD does not affect society equally. Although reports are conflicting [7[7][8][9],8,9], the largest systematic review recently conducted highlights the under-recognition of the disease in women, with PAD more prevalent in women >25 years of age in high-income countries. Further, a higher proportion of women with PAD remain asymptomatic [10[10][11],11], which is associated with delayed presentation [12] and worse clinical outcomes post intervention [13,14][13][14].
The endothelium is a monolayer of endothelial cells (ECs) which lines the entire vascular tree, playing a critical role in maintaining cardiovascular homeostasis including regulating permeability, blood flow, vessel tone, inflammation, platelet function, and angiogenesis.
2. Sexual Dimorphisms in EC Functions(s) in PAD
A summary of EC function(s) that could impact female PAD pathophysiology described by experimental models is shown in Figure 1. Clinical observations that may reflect differences in EC function(s) in female PAD patients are described in Figure 2. Both experimental and clinical findings are detailed in the text below.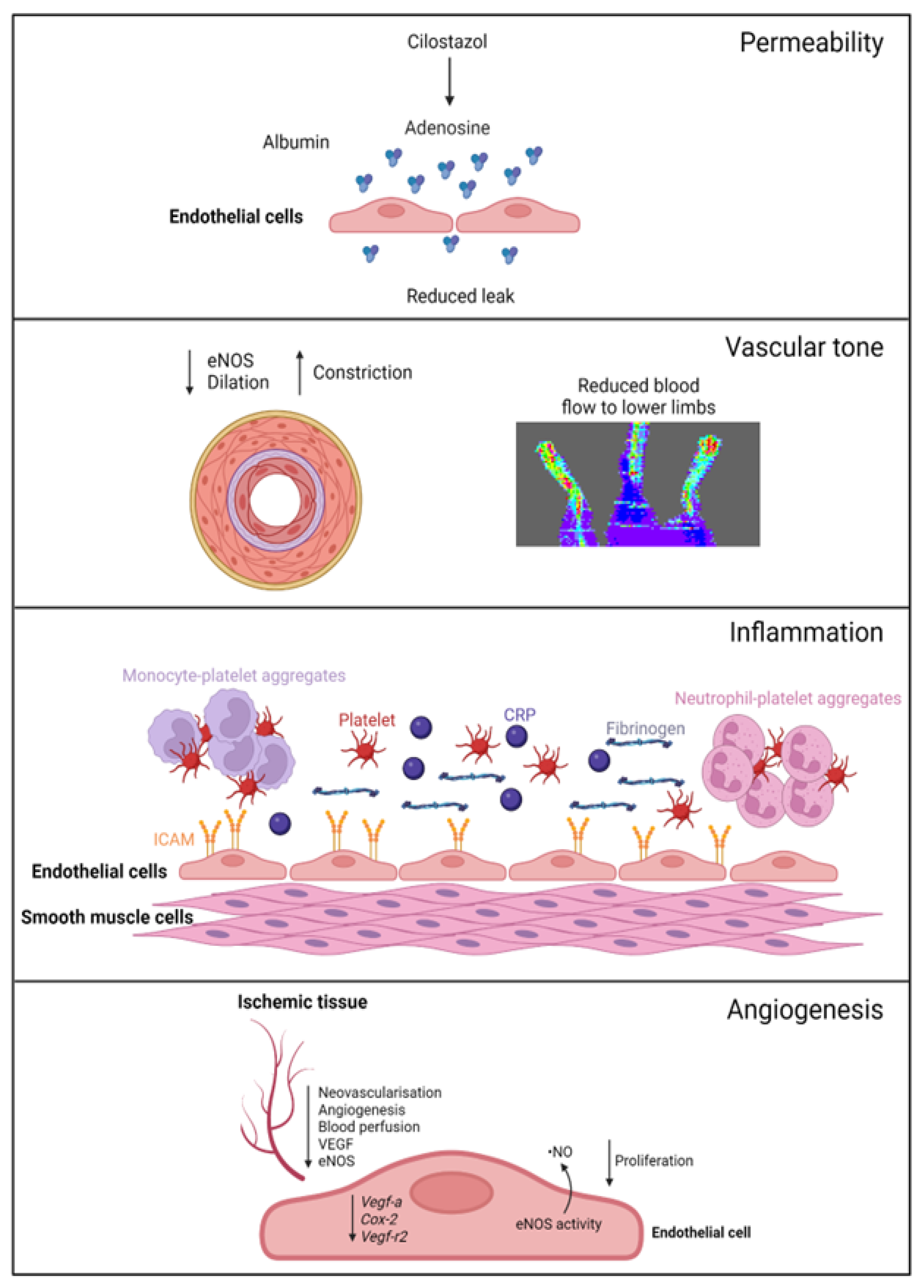
Figure 1. Female-specific findings from experimental models. Permeability: cilostazol increases adenosine levels, reducing leak in female, but not male, vessels. Vascular tone: female ischemic limbs express less eNOS (endothelial nitric oxide synthase) and have reduced arterial relaxation and increased arterial constriction compared to male ischemic limbs. Inflammation: female ECs have increased ICAM (intracellular adhesion molecule 1) expression. Females with PAD have increased levels of platelets, leukocyte–platelet aggregates, CRP (C-reactive protein), and fibrinogen in their circulation. Angiogenesis: female ECs have reduced proliferation and reduced expression of genes regulating angiogenesis, with angiogenic sprouting and migration in female cells specifically reliant on increased eNOS activity and ·NO (nitric oxide) release. Ischemic limbs in female mice have reduced expression of eNOS and VEGF (vascular endothelial growth factor) in tissues, with reduced angiogenesis and neovascularization. Created with BioRender.com (accessed on 9 December 2023).
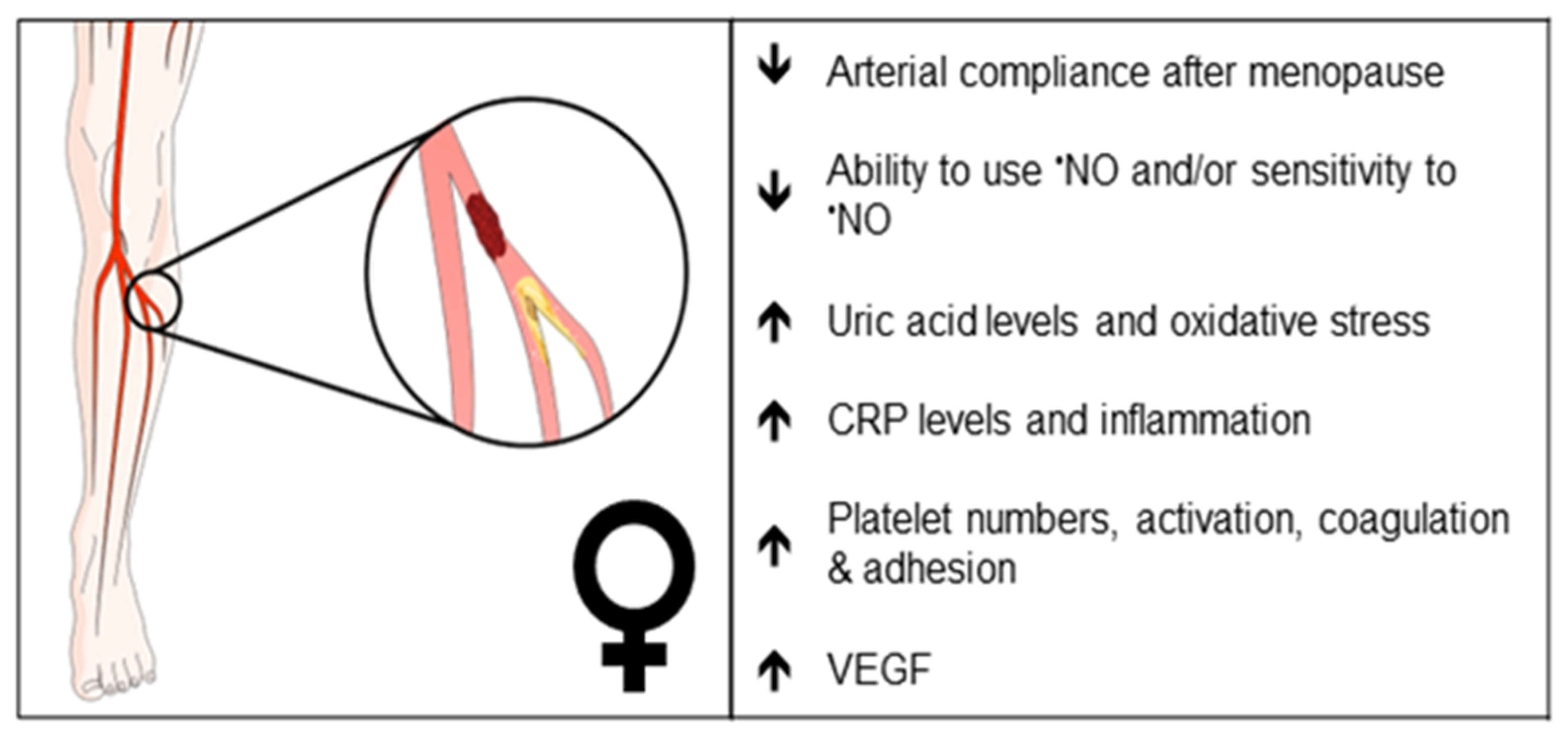
Figure 2. Clinical observations that may reflect differences in endothelial function in female PAD patients. Arrows indicate increase or decrease. •NO, nitric oxide; CRP, C-reactive protein; VEGF, vascular endothelial growth factor.
2.1. Pathogenesis
PAD is traditionally thought to be caused by atherosclerosis; however, a recent study reported that 66% of large peripheral arteries examined from patients with chronic limb-threatening ischemia (CLTI), the severe form of PAD, were blocked by thrombus, in the absence of significant atherosclerosis [17,18][15][16]. Thrombi within smaller vessels were also identified [17,18][15][16]. Thrombosis may play a role in determining PAD disparities, since women have a higher platelet count than males, and female platelets have a higher reactivity than males [19][17]. Women also have more lesions in smaller vessels and multilevel disease [13]. Peripheral arteries from patients present with greater medial calcification and calcified nodules [17,18][15][16]; these may promote rupture by disrupting the fibrous cap. Interestingly, calcified nodules were present in 8% of coronary plaques from women ≥50 years of age vs. 3% of men in the same age bracket [20][18], and it is tempting to speculate that sex differences in calcification in peripheral arteries may exist; however, the authors found no differences in relation to sex. Additional studies are needed to understand the impact of sex and its role in atherosclerosis and/or thrombosis and in microvascular and multi-level disease.2.2. Biomechanical Considerations
Blood vessels from women tend to be smaller in diameter than those of men, including blood vessels of the leg such as the common femoral artery [1] and vein [2], even when corrected for body weight. EC size may impact the vessel diameter; however, studies are conflicting. For example, male lung ECs isolated from mice were smaller than female ECs, whereas male ECs isolated from rat aorta were larger than the corresponding ECs from female rats [3,4][3][4]. Importantly, the smaller vessel size in females may contribute to arterial shear stress, which can influence vascular remodeling, restenosis after revascularization, and arterial compliance [5,6,7][5][6][7]. The smaller vessel size in women may also contribute to difficulties in revascularization [8], increased complications, and mortality. This is particularly evident following percutaneous coronary intervention and bypass grafting in women with coronary artery disease (CAD) [9,10][9][10]. The same may hold true for PAD. Women with CLTI have an increased rate of major adverse cardiovascular events and increased mortality after surgical revascularization or amputation [11,12][11][12]. Differences in sex hormones may also affect arterial compliance, with post-menopausal females having increased arterial stiffness compared to males [13], a finding that is further exaggerated in conditions such as metabolic syndrome [14]. Arterial stiffness contributes to the increased prevalence of hypertension in women [15][19], and hypertension is a risk factor for PAD [16][20]. These differences in the biomechanical properties of arteries may affect the sex-dependent outcomes of PAD treatment. Whether altered EC functions contribute to differential response and recovery post revascularization or surgery in women with PAD, particularly post-menopause women, remains unclear.2.3. Vascular Tone
Sex differences in endothelial-dependent vasodilation are apparent [21], with young healthy female arteries showing increased arterial dilator responses to flow-mediated dilation (FMD; a surrogate of endothelial function) and chemical stimuli through enhanced nitric oxide- (·NO), cyclooxygenase- (COX), and/or hyperpolarization-dependent pathways [22]. In part, this is attributed to increased levels of estrogen during the menstrual cycle. Interestingly, female vessels show greater dependence on ·NO-mediated arterial relaxation, but with aging and loss of estrogen (i.e., menopause), these responses are lost (extensively reviewed in [23,24][23][24]). Table 1, summarizes sex-dependent mechanisms mediating vessel tone in multiple vascular beds isolated from wildtype C57Bl6 mice. In preclinical PAD, using the hindlimb ischemia model, ischemic female limbs had reduced endothelial nitric oxide synthase (eNOS) protein expression, associating with decreased arterial relaxation to acetylcholine, greater resistance to flow, and increased arterial constriction when compared to male limbs [25]. Ischemic female limbs also had reduced blood perfusion to the lower limbs [25]. In humans, systemic ·NO synthesis rates were significantly lower in PAD patients (stage II–IV) compared to control subjects, but not significantly different between sexes [26]. Interestingly, nitrate and cyclic guanosine monophosphate (cGMP) excretion rates were higher in female than in male subjects (with the trend reaching statistical significance), implying altered ·NO signaling in women with PAD, a finding not further elaborated upon by the authors [26]. These studies suggest that females may produce sufficient ·NO but are unable to utilize it for vasodilatory purposes or that the sensitivity of female vessels to ·NO is reduced. Changes in oxidative stress may also contribute to vessel tone. Uric acid is an end-product of purine metabolism and the most plentiful antioxidant in plasma. High levels of uric acid are associated with cardiovascular diseases including PAD [27,28][27][28] and with endothelial dysfunction, in part, by reducing ·NO bioavailability [28,29][28][29]. Taher and colleagues performed a retrospective cross-sectional analysis on peripheral microvascular dysfunction (reactive hyperemia peripheral arterial tonometry via Endo-PAT) and serum uric acid levels ≥5 mg/dL in cardiovascular disease patients. The authors identified a significant positive association between the two, and specifically, the association was only observed in women [30]. Whether this is a possible mechanism for impaired ·NO bioavailability in women with PAD remains to be demonstrated.Table 1.
Sex differences in the mechanisms of relaxation in C57Bl6 mice.
| Stimulus | Age | Gender | Artery | Mechanism of Relaxation | Ref. |
|---|---|---|---|---|---|
| Ach | 8 Weeks | Male | Mesenteric (150 µm) |
~60% ·NO-/COX-dependent ~20% IKca-/SKca-dependant ~20% Unknown |
[31] |
| 8 Weeks | Female | Mesenteric (150 µm) |
~40% ·NO-/COX-dependent ~40% IKca-/SKca-dependant ~20% Undetermined |
[31] | |
| 6–8 Weeks | Male | Superior mesenteric | ~70% ·NO-/COX-dependent ~30% Undetermined |
[32] | |
| Any | Female | * Superior mesenteric | No data | ||
| 6–8 Weeks | Male | Thoracic aorta | 100% ·NO-dependent | [32] | |
| 8 Weeks | Female | Thoracic aorta | 100% ·NO-dependent | [33] | |
| Not specified | Male | Carotid | ~50% ·NO-/COX-dependent ~50% Undetermined |
[34] | |
| 19–23 weeks | Female | Carotid | 100% ·NO-dependent | [35] | |
| Not specified | Male | Femoral | ~60% ·NO-/COX-dependent ~40% Undetermined |
[34] | |
| 19–23 weeks | Female | Femoral | ~75% ·NO ~25% BKca/IKca/SKca |
[35] | |
| Flow | 10–14 weeks | Male | Cerebral | ~50% ·NO-dependent ~50% H2O2-dependent |
[36] |
| Any | Female | * Cerebral | No Data | ||
| 5–6 months | Male | Mesenteric (200 µm) |
~50% ·NO-dependent ~50% Undetermined |
[37] | |
| Any | Female | * Mesenteric | No data | ||
| 26 months | Male | Femoral | ~100% ·NO-dependent | [38] | |
| Any | Male | * Femoral | No data |
Ach, acetylcholine; ·NO, nitric oxide; COX, cyclooxygenase; IKca, intermediate-conductance calcium-activated potassium channel; SKca, small-conductance calcium-activated potassium channel; BKca, big-conductance calcium-activated potassium channel. * No data found in the literature assessing relaxation in these arteries.
2.4. Barrier Function and Permeability
The evidence that barrier function differs in males and females with PAD is weak. However, there are two strands of evidence that point to altered features depending on sex. A recent study examined male and female CAD patients; both sexes had significantly impaired perfusion in their microvasculature, measured as a percentage of microvascular vessels occupied by red blood cells [39]. Females had deeper penetration of red blood cells into the sublingual glycocalyx, indicating greater impairment in barrier function when compared to males [39]. Whether a similar disfunction is observed in women with PAD is yet to be established. Phosphodiesterases (PDEs) play an important role in barrier function, as they inactivate the cyclic nucleotide messengers cyclic adenosine monophosphate (cAMP) and cGMP. ECs are known to express five PDEs, namely, PDE1, PDE2, PDE3, PDE4, and PDE5. Cilostazol is a PDE3 inhibitor, and an anti-platelet medication used to relieve PAD patients with symptoms of intermittent claudication. Cilostazol treatment was shown to improve walking distance [40], in part, via its ability to act as a vasodilator. It can also increase adenosine concentrations in patients with acute coronary syndromes [41] and reduce permeability [42], and, particularly relevant to this review, it reduced permeability in female, but not in male, microvascular ECs [43]. Interestingly, female microvascular ECs express more Pde3b mRNA than male ECs [43], implying that cilostazol may have sex-dependent actions.2.5. Leukocyte Trafficking and Inflammation
How inflammatory cells and molecules relate to sex differences in the presence of PAD is unclear. Sex differences in immune responses have been described [44] and are influenced by the environment, genetic mediators, and hormones. With regard to the latter, the presence of estrogens, particularly, 17β-estradiol in premenopausal women, is thought to be protective [45]. Indeed, premenopausal women have a reduced prevalence of CAD, hypertension, myocardial infarction, and stroke compared to men; however, the prevalence of these conditions in women after menopause surpasses that of men [46]. This protection is in part due to the anti-inflammatory and antioxidant properties of female sex hormones (i.e. estrogens), which are lost after menopause [23]. However, the benefit of estrogen therapy in post-menopausal women is conflicting and reflects the fact that the contribution of sex hormones to inflammation, oxidation, and atherosclerosis is complex and influenced by the effects of the sex chromosomes [47] and age- and sex-dependent differences in specific organs and tissues [23]. What is known is that women with PAD have ~1.4 times higher levels of CRP than men, associating with greater PAD prevalence [48]. Women also have higher levels of CRP and fibrinogen and are more likely to present with CLTI following autogenous vein lower extremity bypass, which is associated with graft failure, with the authors proposing an impaired healing response in women to account for this [49]. Sex differences also appear to impact EC inflammatory marker expression. For example, female skeletal ECs show greater expression of intercellular adhesion molecule-1 (ICAM-1) than male skeletal ECs under basal conditions, whereas vascular cell adhesion molecule (VCAM-1) is expressed in male ECs to a greater extent [50]. This is somewhat supported by other studies; women with PAD of African American descent had elevated levels of ICAM-1, whereas Caucasian women had increased levels of MMP-9 and VCAM-1 when compared to men with PAD from each race [51]. Furthermore, ECs exposed to sera from African American women showed significantly increased intracellular oxidative stress compared to ECs exposed to sera from male African Americans [51]. Importantly, the same authors found that women may have a weakness in their ability to increase capillary blood volume following exercise treatment, since their time to reach minimal calf muscle oxygen saturation levels—an important measure of microcirculatory function—was significantly shorter than men [52]. These findings suggest that anti-inflammatories and medications that improve EC function may be beneficial in women with PAD. In inflammatory and thrombotic conditions, platelet–leukocyte aggregates can form, enhancing platelet activation. A small study identified that the levels of stimulated platelet–neutrophil aggregates were significantly increased in diabetic women with and without cardiovascular disease in comparison to men with the same condition [53]. Increased suppressor of cytokine signaling 3 (SOCS3) expression was also associated with increased circulating monocyte–platelet aggregates in women that had a myocardial infarction [54]. These observations support the notion that increased platelet number and activity, as well as their increased interaction with leukocytes, is linked to increased adhesivity to the endothelium and to increased aggregability. Coagulation factors such as tissue factor can also promote inflammatory responses by activating protease-activated receptors [55]; however, sex-dependent effects are unclear. The crosstalk between inflammation and coagulation factors contributing to PAD pathogenesis, recently reviewed elsewhere [56], highlights the complexity of this system. More studies are needed to fully appreciate the impact of sex-dependent EC thrombo-inflammatory processes in PAD.2.6. Platelets and Coagulation
The endothelium provides a surface for the assembly of platelets and coagulation factors and for the development of thrombosis, which is a common complication of PAD. Females have an overall higher platelet count compared to males, and as mentioned earlier, they have a higher baseline reactivity [19][17]. Female platelets show consistently higher aggregation in response to agonists, such as arachidonic acid, collagen, and adenosine diphosphate (ADP) [57]. In contrast, platelets from healthy female subjects show less baseline platelet adhesion to the endothelium compared to male platelets [58]. This difference in platelet reactivity may be due to sex hormones. Both female and male platelets express receptors for 17β-estradiol as well as androgen and progesterone receptors; however, their effect on platelet function is somewhat controversial [59,60][59][60]. Platelet receptors can also regulate platelet reactivity in a sex-dependent manner. Platelet adhesion and thrombus formation is dependent on platelet glycoprotein receptors including glycoprotein IIb/IIIa (fibrinogen receptor), Iba (von Willebrand factor receptor), and VI and a2b1 (collagen receptors). Platelet activation and aggregation is mediated through the G-protein receptors PAR1 (protease activated receptor 1) and PAR4 (thrombin receptors), P2Y12 receptors (ADP receptors), and the thromboxane A2 receptor, amongst others [61]. While there is no difference in the expression of glycoprotein IIb/IIIa between sexes, women show higher receptor reactivity [59]. Moreover, healthy women and female mice have higher reactivity to PAR1 and PAR4 agonists [62], suggesting sex-dependent expression or activation of these receptors. Circulating coagulation factors are influenced by hormonal differences in females vs. males. The most outstanding example of this is pregnancy, where multiple coagulation factors are upregulated, presumably as an evolutionary mechanism to prevent maternal death from post-partum hemorrhage. Women have higher average levels of von Willebrand factor and factor VIII [63], with further increases in von Willebrand factor and fibrinogen concentrations developing during pregnancy. In contrast, pre-menopausal women have lower levels of the antithrombotic proteins, protein S and protein C, and conversely, lower levels of factor X, an enzyme of the coagulation cascade [64]. Not surprisingly, elevated von Willebrand factor and fibrinogen levels are independently associated with the risk of development of PAD [65]. Fibrinolytic activity is also a feature of PAD with poor outcomes. Women with CAD have high levels of PAI-1 [66], which may indicate impaired fibrinolysis. The increase in PAI-1 was further exaggerated in females with type-2 diabetes [67]. These studies indicate that females with cardiovascular disease, including PAD, have altered platelet function, coagulation, and fibrinolysis, which may contribute to worse outcomes. In terms of current therapies, females display higher baseline platelet reactivity, which contradicts the anti-aggregatory effect of aspirin [19][17]. In a study of low-dose aspirin therapy in unaffected individuals from families with premature coronary disease, women had consistently more reactive platelets compared with men, to multiple agonists at baseline, which persisted after aspirin therapy [19][17]. It must be noted that despite sex differences being statistically significant, the magnitude of the differences was small. Another study with low-dose aspirin, showed that daily aspirin exposure resulted in a paradoxical attenuation of platelet inhibition in response to epinephrine and ADP over time in women but not in men [57]. The second most common anti-platelet agent, clopidogrel, also showed a differential effect in females versus males. Women on clopidogrel, evaluated prior to cardiac surgery, had higher reactivity to ADP compared with men [68]. Inadequate antiplatelet responses to fixed doses in women may support the rationale of sex-tailored agent selection or dosage. In terms of the side effects of anti-platelet treatments, there is some evidence for sex differences; a multivariate analysis confirmed that newer antiplatelet agents are an independent risk factor for bleeding only in women [69]. How these findings relate to EC sex-dependent differences in patients with PAD require further elucidation.2.7. Angiogenesis
Angiogenesis is a critical physiological process in which new blood vessels grow from a pre-existing vessel bed. These neovessels are the cornerstones of nutrient diffusion, essential in tissue development and wound repair. Increasing evidence suggests sex differences in angiogenic responses in ECs. For example, female microvascular ECs isolated from skeletal muscle appeared to grow more slowly than male cells [50]. In a rat cornea model, neovascularization was significantly reduced in females compared to males, across a range of rat strains [70], and this was an androgen-independent response [70]. Rather, the expression of cyclooxygenase-2 (Cox-2), vascular endothelial growth factor-A (VEGF-A), and vascular endothelial growth factor-receptor 2 (VEGF-R2) was higher in male than in female ECs, suggesting enhanced angiogenic priming in male ECs. In porcine valvular ECs, male cells exhibited higher proliferation rates vs. female cells, which was associated with increased secretion of pro-angiogenic VEGF-A, platelet derived growth factor (PDGF), and endothelin-1 from neighboring male interstitial cells, whereas female interstitial cells secreted greater levels of anti-angiogenic factors [71]. In contrast, other studies either reported no differences in angiogenic processes or showed increased angiogenic capacity in female ECs compared to male ECs in vitro, associated with increased platelet endothelial cell adhesion molecule-1 (PECAM-1) or eNOS expression [72,73,74][72][73][74]. Interestingly, female (but not male) EC angiogenic sprouting and migration were found to be reliant on increased eNOS activity and ·NO release [75,76][75][76]. Angiogenesis is considered a key protective mechanism against symptomatic PAD—including against the susceptibility to claudication, ischemic ulcers, and limb amputation. Sex-dependent changes in angiogenesis have been described in mouse models of PAD, where reduced angiogenesis and capillary density were associated with impaired blood perfusion in female vs. male C57Bl6 ischemic hindlimbs [25]. The authors found that the basal VEGF and eNOS protein expression was greater in female limb tissues, whereas 7 days post-ischemia, male tissues displayed greater VEGF and eNOS expression [25]. Sex hormones also play a role, since estrogen-related receptor-α expression is induced in the ischemic hindlimb of male mice, which is associated with increased vascularization and non-leaky blood vessel formation [77]. Further, oophorectomized mice showed reduced neovascularization and eNOS protein expression after hindlimb ischemia when compared to female control mice [78]. A clinical study assessing 234 patients (145 males, 89 females) with PAD and 50 healthy controls reported higher levels of plasma VEGF in female vs. male PAD patients [79]. Given that VEGF is increased in ischemia and high levels of VEGF predict PAD progression and severity [80], disease may indeed be far worse in females.References
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191.
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Prac. 2019, 157, 107843.
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429.
- Aitken, S.J. Peripheral artery disease in the lower limbs: The importance of secondary risk prevention for improved long-term prognosis. Am. J. Gen. Pract. 2022, 49, 239–244.
- Robbins, J.M.; Strauss, G.; Aron, D.; Long, J.; Kuba, J.; Kaplan, Y. Mortality rates and diabetic foot ulcers: Is it time to communicate mortality risk to patients with diabetic foot ulceration? J. Am. Podiatr. Med. Assoc. 2008, 98, 489–493.
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1808–1817.
- Sigvant, B.; Wiberg-Hedman, K.; Bergqvist, D.; Rolandsson, O.; Andersson, B.; Persson, E.; Wahlberg, E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc. Surg. 2007, 45, 1185–1191.
- Moussa, I.D.; Jaff, M.R.; Mehran, R.; Gray, W.; Dangas, G.; Lazic, Z.; Moses, J.W. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: The Peripheral Arterial Disease in Interventional Patients Study. Catheter. Cardiovasc. Interv. 2009, 73, 719–724.
- Diehm, C.; Schuster, A.; Allenberg, J.R.; Darius, H.; Haberl, R.; Lange, S.; Pittrow, D.; von Stritzky, B.; Tepohl, G.; Trampisch, H.J. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis 2004, 172, 95–105.
- McDermott, M.M.; Fried, L.; Simonsick, E.; Ling, S.; Guralnik, J.M. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The women’s health and aging study. Circulation 2000, 101, 1007–1012.
- Arnold, S.F.; Notides, A.C. An antiestrogen: A phosphotyrosyl peptide that blocks dimerization of the human estrogen receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 7475–7479.
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511.
- Choi, K.H.; Park, T.K.; Kim, J.; Ko, Y.G.; Yu, C.W.; Yoon, C.H.; Lee, J.H.; Min, P.K.; Koh, Y.S.; Chae, I.H.; et al. Sex Differences in Outcomes Following Endovascular Treatment for Symptomatic Peripheral Artery Disease: An Analysis From the K- VIS ELLA Registry. J. Am. Heart Assoc. 2019, 8, e010849.
- Lefebvre, K.M.; Chevan, J. The persistence of gender and racial disparities in vascular lower extremity amputation: An examination of HCUP-NIS data (2002-2011). Vasc. Med. 2015, 20, 51–59.
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities between Peripheral Artery Disease and Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1982–1989.
- Narula, N.; Dannenberg, A.J.; Olin, J.W.; Bhatt, D.L.; Johnson, K.W.; Nadkarni, G.; Min, J.; Torii, S.; Poojary, P.; Anand, S.S.; et al. Pathology of Peripheral Artery Disease in Patients with Critical Limb Ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163.
- Becker, D.M.; Segal, J.; Vaidya, D.; Yanek, L.R.; Herrera-Galeano, J.E.; Bray, P.F.; Moy, T.F.; Becker, L.C.; Faraday, N. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006, 295, 1420–1427.
- Yahagi, K.; Davis, H.R.; Arbustini, E.; Virmani, R. Sex differences in coronary artery disease: Pathological observations. Atherosclerosis 2015, 239, 260–267.
- Kavurma, M.M.; Boccanfuso, L.; Cutmore, C.; Passam, F.; Patel, S.; Hennessy, A.; Loa, J.; Figtree, G.A.; Golledge, J.; Robinson, D.A.; et al. A hidden problem: Peripheral artery disease in women. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 342–350.
- Kavurma, M.M.; Bursill, C.; Stanley, C.P.; Passam, F.; Cartland, S.P.; Patel, S.; Loa, J.; Figtree, G.A.; Golledge, J.; Aitken, S.; et al. Endothelial cell dysfunction: Implications for the pathogenesis of peripheral artery disease. Front. Cardiovasc. Med. 2022, 9, 1054576.
- Celermajer, D.S.; Sorensen, K.E.; Spiegelhalter, D.J.; Georgakopoulos, D.; Robinson, J.; Deanfield, J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994, 24, 471–476.
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex differences in the vascular function and related mechanisms: Role of 17beta-estradiol. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1499–H1518.
- Meyer, M.R.; Barton, M. Estrogens and Coronary Artery Disease: New Clinical Perspectives. Adv. Pharmacol. 2016, 77, 307–360.
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588.
- Peng, X.; Wang, J.; Lassance-Soares, R.M.; Najafi, A.H.; Sood, S.; Aghili, N.; Alderman, L.O.; Panza, J.A.; Faber, J.E.; Wang, S.; et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2027–H2034.
- Boger, R.H.; Bode-Boger, S.M.; Thiele, W.; Junker, W.; Alexander, K.; Frolich, J.C. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 1997, 95, 2068–2074.
- Li, Q.; Zhou, Y.; Dong, K.; Wang, A.; Yang, X.; Zhang, C.; Zhu, Y.; Wu, S.; Zhao, X. The Association between Serum Uric Acid Levels and the Prevalence of Vulnerable Atherosclerotic Carotid Plaque: A Cross-sectional Study. Sci. Rep. 2015, 5, 10003.
- Lee, T.S.; Lu, T.M.; Chen, C.H.; Guo, B.C.; Hsu, C.P. Hyperuricemia induces endothelial dysfunction and accelerates atherosclerosis by disturbing the asymmetric dimethylarginine/dimethylarginine dimethylaminotransferase 2 pathway. Redox. Biol. 2021, 46, 102108.
- Hong, Q.; Qi, K.; Feng, Z.; Huang, Z.; Cui, S.; Wang, L.; Fu, B.; Ding, R.; Yang, J.; Chen, X.; et al. Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium 2012, 51, 402–410.
- Taher, R.; Sara, J.D.; Prasad, M.; Kolluri, N.; Toya, T.; Lerman, L.O.; Lerman, A. Elevated serum uric acid is associated with peripheral endothelial dysfunction in women. Atherosclerosis 2019, 290, 37–43.
- Ellis, A.; Cheng, Z.J.; Li, Y.; Jiang, Y.F.; Yang, J.; Pannirselvam, M.; Ding, H.; Hollenberg, M.D.; Triggle, C.R. Effects of a Western diet versus high glucose on endothelium-dependent relaxation in murine micro- and macro-vasculature. Eur. J. Pharmacol. 2008, 601, 111–117.
- Ellis, A.; Pannirselvam, M.; Anderson, T.J.; Triggle, C.R. Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br. J. Pharmacol. 2003, 140, 1193–1200.
- Darblade, B.; Pendaries, C.; Krust, A.; Dupont, S.; Fouque, M.J.; Rami, J.; Chambon, P.; Bayard, F.; Arnal, J.F. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ. Res. 2002, 90, 413–419.
- Gauthier, K.M.; Goldman, D.H.; Aggarwal, N.T.; Chawengsub, Y.; Falck, J.R.; Campbell, W.B. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H725–H735.
- Crauwels, H.M.; Van Hove, C.E.; Herman, A.G.; Bult, H. Heterogeneity in relaxation mechanisms in the carotid and the femoral artery of the mouse. Eur. J. Pharmacol. 2000, 404, 341–351.
- Drouin, A.; Thorin, E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke 2009, 40, 1827–1833.
- Favre, J.; Vessieres, E.; Guihot, A.L.; Proux, C.; Grimaud, L.; Rivron, J.; Garcia, M.C.; Rethore, L.; Zahreddine, R.; Davezac, M.; et al. Membrane estrogen receptor alpha (ERalpha) participates in flow-mediated dilation in a ligand-independent manner. eLife 2021, 10, e68695.
- Diaz, M.; Parikh, V.; Ismail, S.; Maxamed, R.; Tye, E.; Austin, C.; Dew, T.; Graf, B.A.; Vanhees, L.; Degens, H.; et al. Differential effects of resveratrol on the dilator responses of femoral arteries, ex vivo. Nitric. Oxide 2019, 92, 1–10.
- Brands, J.; Hubel, C.A.; Althouse, A.; Reis, S.E.; Pacella, J.J. Noninvasive sublingual microvascular imaging reveals sex-specific reduction in glycocalyx barrier properties in patients with coronary artery disease. Physiol. Rep. 2020, 8, e14351.
- Pande, R.L.; Hiatt, W.R.; Zhang, P.; Hittel, N.; Creager, M.A. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc. Med. 2010, 15, 181–188.
- Xue, Y.; Wang, Z.; Wu, H.; Li, X.; Chen, J.; Lv, Q. Cilostazol increases adenosine plasma concentration in patients with acute coronary syndrome. J. Clin. Pharm. Ther. 2021, 46, 328–332.
- Edrissi, H.; Schock, S.C.; Cadonic, R.; Hakim, A.M.; Thompson, C.S. Cilostazol reduces blood brain barrier dysfunction, white matter lesion formation and motor deficits following chronic cerebral hypoperfusion. Brain Res. 2016, 1646, 494–503.
- Wang, J.; Bingaman, S.; Huxley, V.H. Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1146–H1154.
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638.
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33.
- Crandall, C.J.; Barrett-Connor, E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: A systematic review. Endocrinol. Metab. Clin. N. Am. 2013, 42, 227–253.
- Alsiraj, Y.; Thatcher, S.E.; Blalock, E.; Fleenor, B.; Daugherty, A.; Cassis, L.A. Sex Chromosome Complement Defines Diffuse Versus Focal Angiotensin II-Induced Aortic Pathology. Arter. Thromb. Vasc. Biol. 2018, 38, 143–153.
- Hiramoto, J.S.; Katz, R.; Weisman, S.; Conte, M. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. J. Am. Heart Assoc. 2014, 3, e000651.
- Hiramoto, J.S.; Owens, C.D.; Kim, J.M.; Boscardin, J.; Belkin, M.; Creager, M.A.; Conte, M.S. Sex-based differences in the inflammatory profile of peripheral artery disease and the association with primary patency of lower extremity vein bypass grafts. J. Vasc. Surg. 2012, 56, 387–395; discussion 395.
- Huxley, V.H.; Kemp, S.S.; Schramm, C.; Sieveking, S.; Bingaman, S.; Yu, Y.; Zaniletti, I.; Stockard, K.; Wang, J. Sex differences influencing micro- and macrovascular endothelial phenotype in vitro. J. Physiol. 2018, 596, 3929–3949.
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Sosnowska, D.; Casanegra, A.I.; Ungvari, Z.; Csiszar, A.; Sonntag, W.E. Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2015, 61, 1249–1257.
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S. Sex-specific predictors of improved walking with step-monitored, home-based exercise in peripheral artery disease. Vasc. Med. 2015, 20, 424–431.
- Tuttle, H.A.; Davis-Gorman, G.; Goldman, S.; Copeland, J.G.; McDonagh, P.F. Platelet-neutrophil conjugate formation is increased in diabetic women with cardiovascular disease. Cardiovasc. Diabetol. 2003, 2, 12.
- Barrett, T.J.; Schlegel, M.; Zhou, F.; Gorenchtein, M.; Bolstorff, J.; Moore, K.J.; Fisher, E.A.; Berger, J.S. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci. Transl. Med. 2019, 11, eaax0481.
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303.
- Miceli, G.; Basso, M.G.; Rizzo, G.; Pintus, C.; Tuttolomondo, A. The Role of the Coagulation System in Peripheral Arterial Disease: Interactions with the Arterial Wall and Its Vascular Microenvironment and Implications for Rational Therapies. Int. J. Mol. Sci. 2022, 23, 14914.
- Friede, K.A.; Infeld, M.M.; Tan, R.S.; Knickerbocker, H.J.; Myers, R.A.; Dubois, L.G.; Thompson, J.W.; Kaddurah-Daouk, R.; Ginsburg, G.S.; Ortel, T.L.; et al. Influence of Sex on Platelet Reactivity in Response to Aspirin. J. Am. Heart Assoc. 2020, 9, e014726.
- Escolar, G.; Bastida, E.; Garrido, M.; Rodriguez-Gomez, J.; Castillo, R.; Ordinas, A. Sex-related differences in the effects of aspirin on the interaction of platelets with subendothelium. Thromb. Res. 1986, 44, 837–847.
- Gasperi, V.; Catani, M.V.; Savini, I. Platelet Responses in Cardiovascular Disease: Sex-Related Differences in Nutritional and Pharmacological Interventions. Cardiovasc. Ther. 2020, 2020, 2342837.
- Nakano, Y.; Oshima, T.; Matsuura, H.; Kajiyama, G.; Kambe, M. Effect of 17beta-estradiol on inhibition of platelet aggregation in vitro is mediated by an increase in NO synthesis. Arter. Thromb. Vasc. Biol. 1998, 18, 961–967.
- Cassar, K.; Bachoo, P.; Brittenden, J. The role of platelets in peripheral vascular disease. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 6–15.
- Soo Kim, B.; Auerbach, D.S.; Sadhra, H.; Godwin, M.; Bhandari, R.; Ling, F.S.; Mohan, A.; Yule, D.I.; Wagner, L., 2nd; Rich, D.Q.; et al. Sex-Specific Platelet Activation Through Protease-Activated Receptors Reverses in Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2021, 41, 390–400.
- Roy-O’Reilly, M.; McCullough, L.D. Sex differences in stroke: The contribution of coagulation. Exp. Neurol. 2014, 259, 16–27.
- Henkens, C.M.; Bom, V.J.; Van der Schaaf, W.; Pelsma, P.M.; Sibinga, C.T.; de Kam, P.J.; van der Meer, J. Plasma levels of protein S, protein C, and factor X: Effects of sex, hormonal state and age. Thromb. Haemost. 1995, 74, 1271–1275.
- Smith, F.B.; Lee, A.J.; Hau, C.M.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. Plasma fibrinogen, haemostatic factors and prediction of peripheral arterial disease in the Edinburgh Artery Study. Blood Coagul. Fibrinolysis 2000, 11, 43–50.
- Ossei-Gerning, N.; Wilson, I.J.; Grant, P.J. Sex differences in coagulation and fibrinolysis in subjects with coronary artery disease. Thromb. Haemost. 1998, 79, 736–740.
- Mansfield, M.W.; Heywood, D.M.; Grant, P.J. Sex differences in coagulation and fibrinolysis in white subjects with non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 160–164.
- Ranucci, M.; Aloisio, T.; Di Dedda, U.; Menicanti, L.; de Vincentiis, C.; Baryshnikova, E.; Surgical and Clinical Outcome REsearch (SCORE) Group. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS ONE 2019, 14, e0225771.
- Grodecki, K.; Huczek, Z.; Scislo, P.; Kowara, M.; Raposeiras-Roubin, S.; D’Ascenzo, F.; Abu-Assi, E.; Henriques, J.P.S.; Saucedo, J.; Gonzalez-Juanatey, J.R.; et al. Gender-related differences in post-discharge bleeding among patients with acute coronary syndrome on dual antiplatelet therapy: A BleeMACS sub-study. Thromb. Res. 2018, 168, 156–163.
- Irani, Y.D.; Pulford, E.; Mortimer, L.; Irani, S.; Butler, L.; Klebe, S.; Williams, K.A. Sex differences in corneal neovascularization in response to superficial corneal cautery in the rat. PLoS ONE 2019, 14, e0221566.
- Nelson, V.; Patil, V.; Simon, L.R.; Schmidt, K.; McCoy, C.M.; Masters, K.S. Angiogenic Secretion Profile of Valvular Interstitial Cells Varies with Cellular Sex and Phenotype. Front. Cardiovasc. Med. 2021, 8, 736303.
- Dadwal, U.C.; Bhatti, F.U.R.; Awosanya, O.D.; Nagaraj, R.U.; Perugini, A.J., 3rd; Sun, S.; Valuch, C.R.; de Andrade Staut, C.; Mendenhall, S.K.; Tewari, N.P.; et al. The effects of bone morphogenetic protein 2 and thrombopoietin treatment on angiogenic properties of endothelial cells derived from the lung and bone marrow of young and aged, male and female mice. FASEB J. 2021, 35, e21840.
- Zhang, Y.; Dong, X.; Shirazi, J.; Gleghorn, J.P.; Lingappan, K. Pulmonary endothelial cells exhibit sexual dimorphism in their response to hyperoxia. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1287–H1292.
- Boscaro, C.; Trenti, A.; Baggio, C.; Scapin, C.; Trevisi, L.; Cignarella, A.; Bolego, C. Sex Differences in the Pro-Angiogenic Response of Human Endothelial Cells: Focus on PFKFB3 and FAK Activation. Front. Pharmacol. 2020, 11, 587221.
- Cattaneo, M.G.; Banfi, C.; Brioschi, M.; Lattuada, D.; Vicentini, L.M. Sex-dependent differences in the secretome of human endothelial cells. Biol. Sex Differ. 2021, 12, 7.
- Cattaneo, M.G.; Vanetti, C.; Decimo, I.; Di Chio, M.; Martano, G.; Garrone, G.; Bifari, F.; Vicentini, L.M. Sex-specific eNOS activity and function in human endothelial cells. Sci. Rep. 2017, 7, 9612.
- Sopariwala, D.H.; Likhite, N.; Pei, G.; Haroon, F.; Lin, L.; Yadav, V.; Zhao, Z.; Narkar, V.A. Estrogen-related receptor alpha is involved in angiogenesis and skeletal muscle revascularization in hindlimb ischemia. FASEB J. 2021, 35, e21480.
- Matsubara, K.; Harada, H.; Ando, N.; Watada, S.; Obara, H.; Matsumoto, K.; Kitagawa, Y. Estrogen deficiency attenuates neovascularization in a murine model of hindlimb ischemia. J. Surg. Res. 2012, 178, 1022–1028.
- Makin, A.J.; Chung, N.A.; Silverman, S.H.; Lip, G.Y. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: A link between angiogenesis and thrombogenesis? Clin. Sci. 2003, 104, 397–404.
- Stehr, A.; Topel, I.; Muller, S.; Unverdorben, K.; Geissler, E.K.; Kasprzak, P.M.; Schlitt, H.J.; Steinbauer, M. VEGF: A surrogate marker for peripheral vascular disease. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 330–332.
More
