Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sian Cartland and Version 2 by Camila Xu.
Peripheral artery disease (PAD) is caused by blocked arteries due to atherosclerosis and/or thrombosis which reduce blood flow to the lower limbs. It results in major morbidity, including ischemic limb, claudication, and amputation, with patients also suffering a heightened risk of heart attack, stroke, and death.
- peripheral artery disease
- endothelial cell dysfunction
- sex differences
1. Introduction
Peripheral artery disease (PAD) is a disease with high human and social impact, significantly reducing the quality of life. In this condition, an impairment of the blood supply due to atherosclerosis results in ischemia, most commonly to the lower limbs. More than 230 million people are affected by PAD globally [1], with the prevalence expected to increase because of a rise in diabetes mellitus [2]. In severe cases, patients, particularly those with diabetes, develop gangrene, which necessitates surgical amputation of the limbs. In the United States alone, 185,000 limbs are amputated every year, and it is estimated that by 2050, >3.5 million U.S. citizens will live without a limb [3]. Although revascularization surgery can improve perfusion, the current interventions may be insufficient because of extensive disease. Furthermore, the underlying atherosclerotic disease remains, and patients with PAD frequently undergo multiple vascular surgical procedures, each of which increases the risk of heart attack and stroke [4]. Indeed, patients who have undergone below- or above-knee amputations are more likely to die within 5 years; a mortality rate greater than those of breast, colon, and prostate cancer [5]. PAD, therefore, causes major trauma and disability, costing the U.S. economy USD 84–380 billion/year [6].
Remarkably, PAD does not affect society equally. Although reports are conflicting [7][8][9][7,8,9], the largest systematic review recently conducted highlights the under-recognition of the disease in women, with PAD more prevalent in women >25 years of age in high-income countries. Further, a higher proportion of women with PAD remain asymptomatic [10][11][10,11], which is associated with delayed presentation [12] and worse clinical outcomes post intervention [13][14][13,14].
The endothelium is a monolayer of endothelial cells (ECs) which lines the entire vascular tree, playing a critical role in maintaining cardiovascular homeostasis including regulating permeability, blood flow, vessel tone, inflammation, platelet function, and angiogenesis.
2. Sexual Dimorphisms in EC Functions(s) in PAD
A summary of EC function(s) that could impact female PAD pathophysiology described by experimental models is shown in Figure 1. Clinical observations that may reflect differences in EC function(s) in female PAD patients are described in Figure 2. Both experimental and clinical findings are detailed in the text below.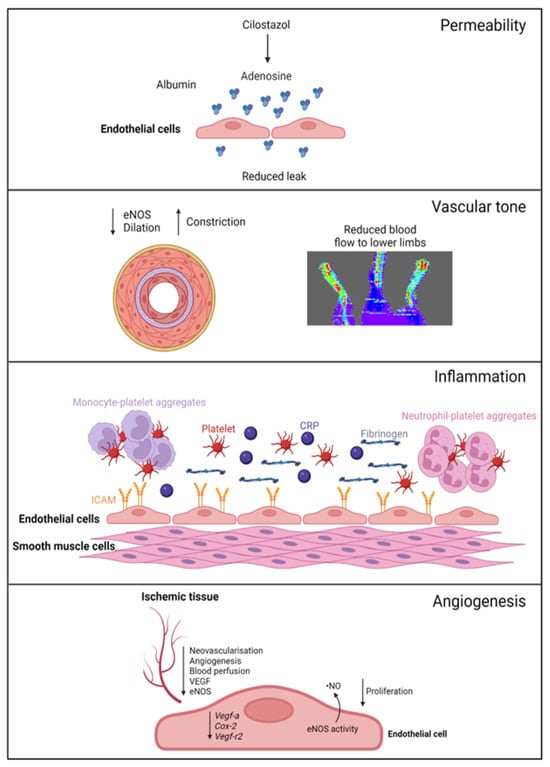
Figure 1. Female-specific findings from experimental models. Permeability: cilostazol increases adenosine levels, reducing leak in female, but not male, vessels. Vascular tone: female ischemic limbs express less eNOS (endothelial nitric oxide synthase) and have reduced arterial relaxation and increased arterial constriction compared to male ischemic limbs. Inflammation: female ECs have increased ICAM (intracellular adhesion molecule 1) expression. Females with PAD have increased levels of platelets, leukocyte–platelet aggregates, CRP (C-reactive protein), and fibrinogen in their circulation. Angiogenesis: female ECs have reduced proliferation and reduced expression of genes regulating angiogenesis, with angiogenic sprouting and migration in female cells specifically reliant on increased eNOS activity and ·NO (nitric oxide) release. Ischemic limbs in female mice have reduced expression of eNOS and VEGF (vascular endothelial growth factor) in tissues, with reduced angiogenesis and neovascularization. Created with BioRender.com (accessed on 9 December 2023).
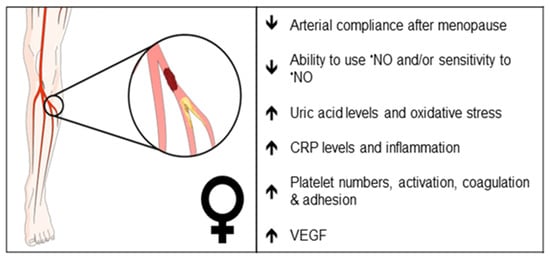
Figure 2. Clinical observations that may reflect differences in endothelial function in female PAD patients. Arrows indicate increase or decrease. •NO, nitric oxide; CRP, C-reactive protein; VEGF, vascular endothelial growth factor.
2.1. Pathogenesis
PAD is traditionally thought to be caused by atherosclerosis; however, a recent study reported that 66% of large peripheral arteries examined from patients with chronic limb-threatening ischemia (CLTI), the severe form of PAD, were blocked by thrombus, in the absence of significant atherosclerosis [15][16][17,18]. Thrombi within smaller vessels were also identified [15][16][17,18]. Thrombosis may play a role in determining PAD disparities, since women have a higher platelet count than males, and female platelets have a higher reactivity than males [17][19]. Women also have more lesions in smaller vessels and multilevel disease [13]. Peripheral arteries from patients present with greater medial calcification and calcified nodules [15][16][17,18]; these may promote rupture by disrupting the fibrous cap. Interestingly, calcified nodules were present in 8% of coronary plaques from women ≥50 years of age vs. 3% of men in the same age bracket [18][20], and it is tempting to speculate that sex differences in calcification in peripheral arteries may exist; however, the authors found no differences in relation to sex. Additional studies are needed to understand the impact of sex and its role in atherosclerosis and/or thrombosis and in microvascular and multi-level disease.2.2. Biomechanical Considerations
Blood vessels from women tend to be smaller in diameter than those of men, including blood vessels of the leg such as the common femoral artery [1] and vein [2], even when corrected for body weight. EC size may impact the vessel diameter; however, studies are conflicting. For example, male lung ECs isolated from mice were smaller than female ECs, whereas male ECs isolated from rat aorta were larger than the corresponding ECs from female rats [3][4][3,4]. Importantly, the smaller vessel size in females may contribute to arterial shear stress, which can influence vascular remodeling, restenosis after revascularization, and arterial compliance [5][6][7][5,6,7]. The smaller vessel size in women may also contribute to difficulties in revascularization [8], increased complications, and mortality. This is particularly evident following percutaneous coronary intervention and bypass grafting in women with coronary artery disease (CAD) [9][10][9,10]. The same may hold true for PAD. Women with CLTI have an increased rate of major adverse cardiovascular events and increased mortality after surgical revascularization or amputation [11][12][11,12]. Differences in sex hormones may also affect arterial compliance, with post-menopausal females having increased arterial stiffness compared to males [13], a finding that is further exaggerated in conditions such as metabolic syndrome [14]. Arterial stiffness contributes to the increased prevalence of hypertension in women [19][15], and hypertension is a risk factor for PAD [20][16]. These differences in the biomechanical properties of arteries may affect the sex-dependent outcomes of PAD treatment. Whether altered EC functions contribute to differential response and recovery post revascularization or surgery in women with PAD, particularly post-menopause women, remains unclear.2.3. Vascular Tone
Sex differences in endothelial-dependent vasodilation are apparent [21], with young healthy female arteries showing increased arterial dilator responses to flow-mediated dilation (FMD; a surrogate of endothelial function) and chemical stimuli through enhanced nitric oxide- (·NO), cyclooxygenase- (COX), and/or hyperpolarization-dependent pathways [22]. In part, this is attributed to increased levels of estrogen during the menstrual cycle. Interestingly, female vessels show greater dependence on ·NO-mediated arterial relaxation, but with aging and loss of estrogen (i.e., menopause), these responses are lost (extensively reviewed in [23][24][23,24]). Table 1, summarizes sex-dependent mechanisms mediating vessel tone in multiple vascular beds isolated from wildtype C57Bl6 mice. In preclinical PAD, using the hindlimb ischemia model, ischemic female limbs had reduced endothelial nitric oxide synthase (eNOS) protein expression, associating with decreased arterial relaxation to acetylcholine, greater resistance to flow, and increased arterial constriction when compared to male limbs [25]. Ischemic female limbs also had reduced blood perfusion to the lower limbs [25]. In humans, systemic ·NO synthesis rates were significantly lower in PAD patients (stage II–IV) compared to control subjects, but not significantly different between sexes [26]. Interestingly, nitrate and cyclic guanosine monophosphate (cGMP) excretion rates were higher in female than in male subjects (with the trend reaching statistical significance), implying altered ·NO signaling in women with PAD, a finding not further elaborated upon by the authors [26]. These studies suggest that females may produce sufficient ·NO but are unable to utilize it for vasodilatory purposes or that the sensitivity of female vessels to ·NO is reduced. Changes in oxidative stress may also contribute to vessel tone. Uric acid is an end-product of purine metabolism and the most plentiful antioxidant in plasma. High levels of uric acid are associated with cardiovascular diseases including PAD [27][28][27,28] and with endothelial dysfunction, in part, by reducing ·NO bioavailability [28][29][28,29]. Taher and colleagues performed a retrospective cross-sectional analysis on peripheral microvascular dysfunction (reactive hyperemia peripheral arterial tonometry via Endo-PAT) and serum uric acid levels ≥5 mg/dL in cardiovascular disease patients. The authors identified a significant positive association between the two, and specifically, the association was only observed in women [30]. Whether this is a possible mechanism for impaired ·NO bioavailability in women with PAD remains to be demonstrated.Table 1.
Sex differences in the mechanisms of relaxation in C57Bl6 mice.
| Stimulus | Age | Gender | Artery | Mechanism of Relaxation | Ref. |
|---|---|---|---|---|---|
| Ach | 8 Weeks | Male | Mesenteric (150 µm) |
~60% ·NO-/COX-dependent ~20% IKca-/SKca-dependant ~20% Unknown |
[31] |
| 8 Weeks | Female | Mesenteric (150 µm) |
~40% ·NO-/COX-dependent ~40% IKca-/SKca-dependant ~20% Undetermined |
[31] | |
| 6–8 Weeks | Male | Superior mesenteric | ~70% ·NO-/COX-dependent ~30% Undetermined |
[32] | |
| Any | Female | * Superior mesenteric | No data | ||
| 6–8 Weeks | Male | Thoracic aorta | 100% ·NO-dependent | [32] | |
| 8 Weeks | Female | Thoracic aorta | 100% ·NO-dependent | [33] | |
| Not specified | Male | Carotid | ~50% ·NO-/COX-dependent ~50% Undetermined |
[34] | |
| 19–23 weeks | Female | Carotid | 100% ·NO-dependent | [35] | |
| Not specified | Male | Femoral | ~60% ·NO-/COX-dependent ~40% Undetermined |
[34] | |
| 19–23 weeks | Female | Femoral | ~75% ·NO ~25% BKca/IKca/SKca |
[35] | |
| Flow | 10–14 weeks | Male | Cerebral | ~50% ·NO-dependent ~50% H2O2-dependent |
[36] |
| Any | Female | * Cerebral | No Data | ||
| 5–6 months | Male | Mesenteric (200 µm) |
~50% ·NO-dependent ~50% Undetermined |
[37] | |
| Any | Female | * Mesenteric | No data | ||
| 26 months | Male | Femoral | ~100% ·NO-dependent | [38] | |
| Any | Male | * Femoral | No data |
Ach, acetylcholine; ·NO, nitric oxide; COX, cyclooxygenase; IKca, intermediate-conductance calcium-activated potassium channel; SKca, small-conductance calcium-activated potassium channel; BKca, big-conductance calcium-activated potassium channel. * No data found in the literature assessing relaxation in these arteries.
