Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Françoise Corbineau.
Seeds are classified as either: orthodox, seeds that tolerate dehydration; recalcitrant, seeds that are high in moisture content and cannot withstand intensive desiccation; or intermediate, seeds that survive dehydration but die during dry storage at low temperatures. Seed lifespan depends on the seed category and also varies from one species to another. The rate of loss of vigor and viability of orthodox seeds depends mainly on temperature and seed moisture content (MC); the lower the MC and storage temperature, the longer the longevity. Ultimately, storage in liquid nitrogen or seed ultra-drying by well-adapted processes should allow for long-term storage.
- seed longevity
- orthodox
- recalcitrant and intermediate seeds
- ageing
- regulation of ageing
- markers of ageing
- seed banks
1. Introduction
Seeds are remarkable organs that are essential in the life of humans and animals since they ensure plant reproduction. They are the natural forms of preservation of higher plants, designed to perpetuate the species through the germination process. They are also used as direct sources of food or in various industries such as oil, starch, and flour manufacturing and malting. Seed storage is the best technology for preserving and conserving plant biodiversity. For agronomical use, seeds must be stored under conditions that maintain high seed quality (i.e., viability and vigor) [1,2,3,4,5,6,7][1][2][3][4][5][6][7].
Decline in seed viability and seed quality (i.e., germinability and vigor) depends on their tolerance to dehydration and is regulated by three important factors: seed moisture content in equilibrium with the relative humidity of the atmosphere, the storage temperature, and the gaseous environment [1,4,5,6,7,8,9,10,11,12][1][4][5][6][7][8][9][10][11][12]. For the majority of the species classified as orthodox (i.e., seeds that tolerate dehydration [13]), a study found that seed longevity increased when reducing the seed moisture content and decreasing the temperature [1,2,7,10,11,14,15][1][2][7][10][11][14][15]. However, for species classified as recalcitrant, i.e., seeds that do not tolerate dehydration [13[13][14][16],14,16], seed longevity is generally short—from a few weeks to a few months. Such seeds must be stored at high moisture content (between a 20 and 70% fresh weight basis) and at a temperature between 7 and 17 °C and −3 and 5 °C for species of tropical origin and those of temperate climate origin, respectively [17].
Oxidative damage due to ROS’ (reactive oxygen species) reactivity towards cell macromolecules, including proteins, sugars, lipids, and nucleic acids, has been demonstrated to be associated with ageing [1,3,7,12,18,19,20,21,22,23,24,25][1][3][7][12][18][19][20][21][22][23][24][25]. However, a better understanding of the cellular, biochemical, and molecular mechanisms associated with ageing could lead to the identification of new markers of the loss of viability during storage.
2. Biological Categories of Seeds
There exist two main biological types of seeds that have been termed “orthodox” and “recalcitrant” seeds [13]. The majority of seeds referred to as orthodox require desiccation tolerance during their development, allowing them to be stored for long periods under air-dry storage [3,26][3][26]. On the other hand, recalcitrant seeds have a high moisture content at shedding and do not tolerate desiccation; therefore, they are able to be stored in a dry state [18,27,28,29][18][27][28][29]. More recently, a third category of seeds, named “intermediate”, has been defined [30,31][30][31]; they survive the loss of water, but they become damaged and die during dry storage at low temperatures. The Compendium of Information on Seed Storage Behaviour [17,32][17][32] recognizes these three biological types. “Orthodox” seeds generally undergo dehydration prior to shedding; they are desiccation tolerant and can be stored successfully in a dehydrated state at a temperature below freezing for very long periods (decades or longer). At maturity (or harvest), they contain no more than 10–15% water and survive drying to very low moisture content (3–5%). They therefore tolerate subsequent storage at sub-zero temperatures [33]. Orthodox seeds acquire desiccation tolerance relatively early during their development and usually before the maturation drying phase. Several metabolic changes occur with respect to the protection of seed cells against dehydration damage [18,26,33,34,35][18][26][33][34][35]. In particular, carbohydrate metabolism [36,37][36][37] and specific proteins (dehydrins, late embryogenesis abundant proteins: LEA, and heat shock proteins: HSPs) [33] seem to be involved in this process. Some soluble sugars, such as sucrose and oligosaccharides (raffinose, stachyose, and verbascose), might also play an important part in this process by facilitating the stabilization of lipids and proteins in cell membranes or by promoting the vitrification of water and then the protection of cytosolic structures [35,37,38][35][37][38]. “Recalcitrant” seeds are desiccation intolerant. They are highly hydrated at shedding and do not survive drying below a moisture content between 30–65% depending on the species [39]. They include seeds mostly produced by tropical or subtropical species from fruit crops: Litchi chinensis (litchi), Euphorbia longan (longan), Garcinia mangostana (mangosteen), Mangifera indica (mango), and Nephelium lappaceum (rambutan), species used as beverages: Cacao theobroma (cocoa) and Coffea robusta (coffee), plantation crops: Hevea brasiliensis (rubber), Elaeis guineensis (oil palm), and Cocos nucifera (coconut), and many timber species belonging to the Dipterocarpaceae family (Table 1) [27,39,40,41][27][39][40][41]. They also concern temperate species such as Quercus spp. (oak), Juglans spp. (walnut), Castanea spp. (chestnut), Corylus avellana (filbert), and Salix spp. (willow) (Table 1).Table 1. Examples of recalcitrant seeds from temperate and tropical species. Modified from [27,39,40,41].
| Origine | Species | Family |
|---|---|---|
| Temperate | Acer saccharinum | Sapindaceae |
| Acer pseudoplatanus | Sapindaceae | |
| Aesculus hippocastanum | Hippocastanaceae | |
| Castanea spp. | Fagaceae | |
| Corylus avellana | Corylaceae | |
| Juglans spp. | Juglandaceae | |
| Quercus sp. | Fagaceae | |
| Populus spp. | Salicaceae | |
| Salix spp. | Salicaceae | |
| Tropical | Araucaria spp. | Araucariaceae |
| Avicenia marina | Avicenniaceae | |
| Camellia sinensis | Theaceae | |
| Cocos nucifera | Arecaceae/Palmaceae | |
| Euphorbia longan | Euphorbiaceae/Sapindaceae | |
| Garcinia mangostana | Clusiaceae/Guttiferae | |
| Hevea brasiliensis | Euphorbiaceae | |
| Hopea odorata | Dipterocarpaceae | |
| Litchi chinensis | Sapindaceae | |
| Mangifera indica | Anacardiaceae | |
| Nephelium lappaceum | Sapindaceae | |
| Persea americana | Lauraceae | |
| Shorea roxburghii | Dipterocarpaceae | |
| Shorea talura | Dipterocarpaceae | |
| Symphonia globulefera | Guttifereae | |
| Theobroma cacao | Steruliaceae |
Table 2. Examples of recalcitrant seeds and the moisture content below which they die. Modified from [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
Table 3. Examples of cold-sensitive recalcitrant seeds and the lowest temperature limit that they can tolerate. From [62,63].
Examples of cold-sensitive recalcitrant seeds and the lowest temperature limit that they can tolerate. From [62][63].
| Species | Temperature Limit (°C) | |||
|---|---|---|---|---|
| Cedrela odorata (Spanish cedar) | 10 | |||
| Dryobalanops aromatica (Bornean camphol tree) | 5 | |||
| Hevea brasiiensis (rubber) | 15–16 | |||
| [ | 48 | , | ||
| Hopea odorata (Chengal pasir) | 49 | ][ | 12 | 48][49] |
| Euphorbia longan (longan) | 25–30 | [50] | ||
| Hevea brasiliensis | ||||
| Mangifera indica (mango tree) | 12 | (hevea) | 20–25 | [51] |
| Shorea roxburghii (Lac tree) | 12–15 | Hopea odorata | 20–25 | [52] |
| Shorea talura (Jalari tree) | 4 | Litchi chinensis (litchi) | 20–30 | |
| Symphonia globulifera (Buckwax tree) | [ | 48,50,53][ | 15 | 48][50][53] |
| Mangifera indica (mango tree) | 30–35 | [50,52,54][50][52][54] | ||
| Quercus petraea (sessile oak) | 30–60 | [55] | ||
| Quercus robur (pedunculate oak) | 30–48 | [56,57][56][57] | ||
| Quercus rubra (Red oak) | 60–75 | [55] | ||
| Shorea roxburghii | 17–30 | [52] | ||
| Symphonia globulifera | 37–40 | [52,58][52][58] | ||
| Theobroma cacao (cocoa tree) | 45–50 | [59,60][59][60] |
3. The Lifespan of Seeds
The lifespan or longevity of seeds varies greatly depending on the species; some survive for very long periods of time in the soil or under ambient conditions whereas others die rapidly—surviving less than 3 years. Ewart [70] in a treatise entitled “On the longevity of seeds” arbitrarily divided seeds into three classes according to their period of survival under natural conditions: macrobiotic, mesobiotic, and microbiotic seeds. Macrobiotic seeds are defined as seeds capable of surviving for more than 15 years in the soil or under ambient conditions, but seeds with longevity exceeding 50 or 100 years are far from rare. They are often hard seeds (i.e., seeds with seed coats impermeable to water). This has been shown by Becquerel [71] who succeeded in germinating very old seeds from the collections of the Natural History Museum of Paris and estimated their longevity as 55 years (Melilotus lutea), 63–68 years (Cytisus austriacus, Lavatera pseudo-olbia, Ervum lens, and Trifolium arvense), 81–86 years (Mimosa glomerata, Cytisus biflorus, and Astragalus massiliensis), and a period of 100–158 years (Cassia multijuga and Dioclea pauciflora) [1]. Evidence from hundreds to thousands of years of seeds has been described [1]. Lotus (Nelumbo nucifera, the Indian lotus) seeds are also well known for their longevity of several hundred years [1]. For example, Dum [72] also achieved the germination of Chenopodium album and Spergula arvensis seeds that were about 1700 years old. The literature reveals that the record is held by Lupinus arcticus seeds which successfully germinated despite being more than 10,000 years old [73]. However, such long longevity must be regarded with skepticism without direct dating of the seeds [1,34][1][34]. Radiocarbon dating allows us to determine the age of seeds at about 2000 years for the date (Phoenix dactylifera) [74], at 1300 years for lotus (Nelumbo nucifera) [75], and at 600 years for canna (Canna compacta) [76]. The myth concerning the longevity of mummy grains discovered in Egyptian tombs and supposed to remain able to germinate is in fact a mistake or a hoax [1,34][1][34]. Mesobiotic seeds have a lifespan of 3 to 15 years. The great majority of the species fall under this category [1,77][1][77]. They include species with a longevity of 3–5 years, such as rape, bean, pea, carrot, cyclamen, and nasturtium, or longer (5–15 years) such as celery, sugar-beet, cabbage, chicory, and cereals (wheat, oat, and barley). Microbiotic seeds survive at most 3 years under natural conditions. All recalcitrant seeds (see Table 1) and orthodox oleaginous seeds exhibit such short longevity. In this group, we can cite seeds of vegetables (onion, leek, fennel, and parsley) or ornamental species (dahlia, delphinium, petunia, and viola) [1,77,78][1][77][78]. Although this classification gives information concerning the putative survival behavior of numerous species, it is debatable because it does not take into account the main factors of storage (temperature and seed moisture content). Indeed, seed survival is both genetically and environmentally controlled. Depending on the conditions of storage, microbiotic seeds could become mesobiotic or macrobiotic ones.4. Loss of Seed Viability
4.1. Change in Viability during Storage: Viability Equations
At harvest, the initial seed viability is a product of the seed history through development on the mother plant [1,34][1][34]. Subsequent seed longevity depends on post-harvest treatments (drying, cleaning, sorting, coating, etc.) and the conditions of storage (temperature, moisture content, and oxygen availability) [34]. The viability equations are based on fitting a negative cumulative normal distribution to viability percentages. The conversion of the negatively sigmoidal curve obtained to probits linearizes the curve [1,2,34,78][1][2][34][78] (Figure 1).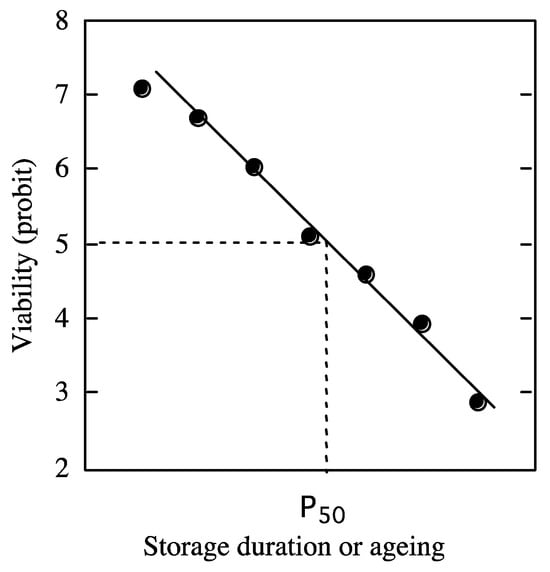
Figure 1.
Theoretical curve of loss of viability in probit during dry storage. A probit value of 5 determines the half-viability period. From [77].
v = Ki − p/10
(KE − (Cw × log m) − (CH × t) − (CQ × t2)
Log P
50
= K
v
− C
1
m − C
2t
t
Table 4. Estimated half-viability period (P50) of some cultivated species. The seeds are stored under open storage conditions in a temperate climate where the mean temperature is 10 °C and the average RH is about 60–75%. Modified from [1,77,86][1][77][86].
| Type of Species | Species | P50 (Years) |
|---|---|---|
| Cereals | Avena sativa (oat) | 12.9 |
| Hordeum vulgare (barley) | 7.2 | |
| Triticum aestivum (wheat) | 7.6 | |
| Secale cereale (rye) | 4.5 | |
| Zea mays (corn) | 9.6 | |
| Legumes | Glycine max (soybean) | 3.4 |
| Medicago sativa (lucerne) | 10.5 | |
| Phaseolus vulgaris (French bean) | 15.9 | |
| Pisum sativum (garden pea) | 15.8 | |
| Vicia faba (broad bean) | 15.6 | |
| Other crops | Beta vulgaris (beet) | 16.5 |
| Brassica napus (rape) | 13.9 | |
| Helianthus annuus (sunflower) | 5.4 | |
| Nicotiana tabacum (tobacco) | 10.3 | |
| Allium cepa (onion) | 5.4 | |
| Vegetables | Apium graveolens (celery) | 4.1 |
| Cichorium intybus (endive) | 5.4 | |
| Cucumis sativus (cucumber) | 4.9 | |
| Daucus carota (carrot) | 6.6 | |
| Lactuca sativa (lettuce) | 6.4 | |
| Lycopersicon esculentum (tomato) | 24.5 | |
| Pastinaca sativa (parsnip) | 4.1 | |
| Petroselinum crispum (parsley) | 3.4 |
4.2. Modulation of Viability by Storage Conditions
As indicated by the “improved viability equation”, loss of seed viability is mainly regulated by seed moisture content and temperature during storage. The storage of orthodox seeds follows two rules [1,2,4,7,34,79,80][1][2][4][7][34][79][80]:- -
-
For each 1–2% decrease in seed moisture content (when the MC ranges between 5 and 14%), the seed storage life is doubled;
- -
-
For each 10 °F (5.6 °C) decrease in seed storage temperature (between 0 °C and 50 °C), the seed storage life is doubled.
4.3. Procedures for Long-Term Storage in Genebanks
Improving seed storage techniques is a major research focus of the International Plant Genetic Resources Institute (IPGRI). Guidelines for long-term conservation recommend storage at −20 °C ± 4 °C and 15 ± 3% RH, considered a conventional method [99]. Cryopreservation is also a possible technique to prolong the longevity of orthodox seeds with short lifespans [100]. In contrast, recalcitrant and intermediate seeds require cryopreservation [7,101,102][7][101][102]. To achieve the appropriate moisture content, Kew recommends equilibrating the seeds to 15% RH at 15 °C (www.rbgkew.org.uk/, accessed on 1 November 2023), Ellis et al. [30,84,85][30][84][85] suggest equilibrating the seeds to 10% RH at 20 °C, and Vertucci and Roos [90,103][90][103] propose to equilibrate the seeds at 20–25% RH at storage temperature. Storing seeds in hermetically sealed containers to maintain their water content is also recommended. Most orthodox seeds also show long longevity when ultra-dried or freeze-dried. One advantage of ultra-drying or freeze-drying is that seeds can be stored at room temperature, but they must be maintained under vacuum in tightly closed containers or bags impermeable to water vapor. Table 5 shows that freeze-drying gives excellent results with the seeds of some vegetable species compared to storage under ambient conditions [62,77][62][77]. However, large seeds, such as pea, soybean, and bean, can be damaged by freeze-drying, with cracks in the cotyledons occurring at thawing or during seed re-imbibition. The results obtained with vacuum-dried seeds (seeds dried under vacuum for 3 days at 20 °C) and freeze-dried seeds (seeds immersed in liquid nitrogen and placed under vacuum for 3 days at 20°) and then stored under vacuum in the presence of silica gel for 12–19 months at 5, 20, and 30 °C clearly indicated that the responsiveness of ultra-dried seeds to storage depends on the species and the temperature of storage. Ultra-dried seeds of lettuce and leek remained viable after 12 months of storage although the germination rate of leek seeds and freeze–dried lettuce seeds was slightly reduced. In onion and lamb’s lettuce seeds, ultra-drying resulted in a marked decrease in seed viability after 12 months of storage, and this deleterious effect was reinforced by increasing the temperature of germination. In addition, ultra-dried seeds stored for 12 months were more sensitive to accelerated ageing treatment (40–45 °C, 100% RH) suggesting that the seeds became progressively less vigorous during storage.Table 5. Comparison of the longevity of seeds from some vegetable species stored in the open air or freeze-dried and stored under vacuum at ambient temperature. C, control non-freeze-dried seeds; FD, freeze-dried seeds. Modified from [62,77][62][77].Species Germination (%) after Storage for 0 (Harvest) 4 Years 8 Years 12 Years 20 Years C FD C FD C FD C FD C FD Allium cepa 92 92 8 72 2 74 2 76 0 68 (onion) Asparagus officinale 93 96 0 62 0 60 2 35 0 25 (asparagus) Cichorium intybus 96 90 4 100 0 68 0 65 0 85 (endive) Foeniculum officinale 100 83 4 72 0 50 0 56 0 50 (fennel) Lonicera caprifolium 90 92 60 100 50 78 38 71 32 96 (honeysuckle) Papaver somnifera 92 90 80 100 16 72 0 60 0 80 (opium poppy) Portulaca oleracea 84 100 100 100 69 96 7 95 0 80 (purslane) Trifolium repens 100 100 100 100 90 88 82 81 100 100 (white clover) Valerianella olitoria 88 96 4 100 0 80 0 84 0 72 (lamb’s lettuce)
References
- Priestley, D.A. Seed aging. In Implications for Seed Storage and Persistence in the Soil; Cornell University Press: Ithaca, NY, USA, 1986; p. 304.
- Murdoch, A.J.; Ellis, R.H. Dormancy, Viability and Longevity. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK; CAB International: Oxon, UK, 2000; pp. 183–214.
- McDonald, M.B. Orthodox seed deterioration and its repair. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: New York, NY, USA; London, UK; Oxford, UK, 2004; pp. 273–304.
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255.
- Solberg, S.O.; Yndgaard, F.; Andreasen, C.; Von Bothmer, R.; Loskutov, I.G.; Asdal, A. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007.
- Hay, F.R.; Rezaei, S.; Buitink, J. Seed moisture isotherms, sorption models and longevity. Front. Plant Sci. 2022, 13, 891913.
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed longevity—The evolution of knowledge and a conceptual framework. Plants 2023, 12, 471.
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 39–52.
- Leopold, A.C.; Vertucci, C.W. Moisture as a regulator of physiological reaction in seeds. In Seed moisture; Stanwood, P.C., McDonald, M.B., Eds.; CSSA: Madison, WI, USA, 1989; Volume 14, pp. 51–67.
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of Seeds in a Gene-Bank: Species Characteristics; Cambridge University Press: Cambridge, UK, 2005; Volume 15.
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant Sci. 2010, 179, 565–573.
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026.
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514.
- Hong, T.D.; Ellis, R.H. A Protocol to Determine Seed Storage Behaviour; International Plant Genetic Resources Institute: Rome, Italy, 1996; p. 64.
- Pritchard, H.W.; Dickie, J.B. Predicting seed longevity: The use and abuse of seed viability equations. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; Kew, Royal Botanic Gardens: London, UK, 2003; pp. 653–722.
- Wyse, S.V.; Dickie, J.B. Predicting the global incidence of seed desiccation sensitivity. J. Ecol. 2017, 105, 1082–1093.
- Hong, T.D.; Linington, S.; Ellis, R.H. Seed storage behavior: A compendium. In Handbooks for Genebanks: No 4; International Plant Genetic Resources Institute: Rome, Italy, 1996; p. 105.
- Smith, M.T.; Berjak, P. Deteriorative changes associated with the loss of viability of stored desiccation-tolerant and desiccation-sensitive seeds. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 701–746.
- Walters, C. Understanding the mechanisms and kinetics of seed ageing. Seed Sci. Res. 1998, 8, 223–244.
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237.
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107.
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA alteration and programmed cell death during ageing of sunflower seed. J. Exp. Bot. 2011, 62, 5003–5011.
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533.
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Dennis, E.S.; Peacock, W.J. Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS ONE 2013, 10, e78471.
- Fleming, M.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 1133–1146.
- Vertucci, C.W.; Farrant, J.M. Acquisition and loss of desiccation tolerance. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 237–271.
- King, M.W.; Roberts, E.H. The Storage of Recalcitrant Seeds—Achievements and Possible Approaches; IBPGR: Rome, Italy, 1979; p. 96.
- Pammenter, N.W.; Berjak, P. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Sci. Res. 1999, 9, 13–37.
- Berjak, P.; Pammenter, N.W. Recalcitrant seeds. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; The Haworth Reference Press: New York, NY, USA; London, UK; Oxford, UK, 2004; pp. 305–345.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour? I. Coffee. J. Exp. Bot. 1990, 41, 1167–1174.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour. II. Effects of provenance, immaturity and imbibition on desiccation tolerance in coffee. J. Exp. Bot. 1991, 42, 653–657.
- Hong, T.D.; Linington, S.H.; Ellis, R.H. Compendium of Information on Seed Storage Behaviour; Kew, Royal Botanic Gardens: London, UK, 1998; Volumes 1–2, p. 901.
- Kermode, A.R.; Finch-Savage, B.E. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 149–184.
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994; p. 445.
- Kermode, A.R. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci. Res. 1997, 7, 75–95.
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599.
- Obendorf, R.L. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci. Res. 1997, 7, 63–74.
- Buitink, J.; Hoekstra, F.A.; Leprince, O. Biochemistry and biophysics of tolerance systems. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 293–318.
- Chin, H.F.; Krishnapillay, B.; Stanwood, P.C. Seed moisture: Recalcitrant vs. orthodox seeds. In Seed Moisture; Stanwood, P.C., McDonald, M.B., Eds.; CSSA: Madison, WI, USA, 1989; Volume 14, pp. 15–22.
- Chin, H.F. Production and storage of recalcitrant seeds in the tropics: Seed problems. Acta Hort. 1978, 83, 17–21.
- Baskin, C.C.; Baskin, J.M. Seeds. In Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: London, UK, 1998; p. 666.
- Berjak, P.; Pammenter, N.W. Aspects of our understanding of the biology and responses of non-orthodox seeds. In Progress in Seed Research; Taylor, A.G., Huang, X.L., Eds.; Cornell University Press: Ithaca, NY, USA, 1997; pp. 81–100.
- Dickie, J.B.; May, K.; Morris, S.V.A.; Titley, S.E. The effects of desiccation on seed survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Sci. Res. 1991, 1, 149–162.
- Pukacka, S.; Wojkiewicz, A. Carbohydrate metabolism in Norway maple and sycomore seeds in relation to desiccation tolerance. J. Plant Physiol. 2002, 159, 273–279.
- Kozeko, L.E.; Troyan, V.M. The relationship between the mitotic activity and moisture content of recalcitrant seeds of Acer saccharinum (L.) during maturation, post maturation drying and germination. Seed Sci. Res. 2000, 10, 225–232.
- Tompsett, P.B. Desiccation studies in relation to the storage of Araucaria seed. Ann. Appl. Biol. 1984, 105, 581–586.
- Salmen-Espindola, L.; Noin, M.; Corbineau, F.; Côme, D. Some cellular and metabolic damage induced by desiccation in recalcitrant Araucaria angustifolia embryos. Seed Sci. Res. 1994, 4, 193–201.
- Fu, J.R.; Jin, J.P.; Peng, Y.F.; Xia, Q.H. Desiccation tolerance in two species with recalcitrant seeds: Clausena lansium (Lour.) and Litchi chinensis (Sonn.). Seed Sci. Res. 1994, 4, 257–261.
- Fu, J.R.; Huang, X.M.; Song, S.Q. Manipulation of desiccation-sensitive axes of wampee (Clausena lansium) to facilitate increaded dehydration tolerance. Seed Sci. Res. 2000, 10, 397–400.
- Fu, J.R.; Zhang, B.Z.; Wang, X.F.; Qiao, Y.Z.; Huang, X.L. Physiological studies in desiccation, wet storage and cryopreservation of recalcitrant seeds of three fruit species and their excised embryonic axes. Seed Sci. Tecnol. 1990, 18, 743–754.
- Chin, H.F.; Aziz, M.; Ang, B.B.; Hamzah, S. The effect of moisture and temperature on the ultrastructure and viability of seeds of Hevea brasiliensis. Seed Sci. Technol. 1981, 9, 411–422.
- Corbineau, F.; Côme, D. Storage of recalcitrant seeds of four tropical species. Seed Sci. Technol. 1988, 16, 97–103.
- Ray, P.K.; Sharma, S.B. Growth, maturity, germination and storage of lichi seeds. Sci. Hort. 1987, 33, 213–221.
- Corbineau, F.; Kanté, M.; Côme, D. Seed germination and seedling development in the mango (Mangifera indica L.). Tree Physiol. 1986, 1, 151–160.
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M. Seeds of Forest Broadleaves from Harvest to Sowing; INRA Editions: Paris, France, 1996; p. 294.
- Finch-Savage, W.E. Seed water status and survival in the recalcitrant species Quercus robur L.: Evidence for a critical moisture content. J. Exp. Bot. 1992, 43, 671–679.
- Finch-Savage, W.E.; Blake, P.S. Indeterminate development in desiccation-sensitive seeds of Quercus robur L. Seed Sci. Res. 1994, 4, 127–133.
- Corbineau, F.; Côme, D. Experiments on the storage of seeds and seedlings of Symphonia globulifera L.f. (Guttiferae). Seed Sci. Technol. 1986, 14, 585–591.
- Li, C.; Sun, W.Q. Desiccation sensitivity and activities of free radical-scavenging enzymes in recalcitrant Theobroma cacao seeds. Seed Sci. Res. 1999, 9, 209–218.
- Liang, Y.; Sun, W.Q. Desiccation tolerance of recalcitrant Theobroma cacao embryonic axes: The optimal drying rate and its physiological basis. J. Exp. Bot. 2000, 51, 1911–1919.
- Chin, H.F.; Roberts, E.H. Recalcitrant Crop Seeds; Tropical Press: Kuala Lumpur, Malaysia, 1980; p. 152.
- Côme, D.; Corbineau, F. Storage of seeds. In Improving Postharvest Technologies of Fruits, Vegetables and Ornamentals; Artés, F., Gil, M.I., Conesa, M.A., Eds.; IIR-IIF: Paris, France, 2000; pp. 755–770.
- Côme, D.; Corbineau, F. Les semences et le froid. In Les Végétaux et le Froid; Côme, D., Ed.; Hermann: Paris, France, 1992; pp. 401–461.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Soetisna, U. Seed storage behaviour in Elaeis guineensis. Seed Sci. Res. 1991, 1, 99–104.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Effect of storage temperature and moisture on the germination of papaya seeds. Seed Sci. Res. 1991, 1, 69–72.
- Sacande, M. Stress, Storage & Survival of Neem Seed. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2000; p. 124.
- Dussert, S.; Chabrillance, N.; Engelmann, F.; Hamon, S. Quantitative estimation of seed desiccation sensitivity using a quantal response model: Application to nine species of the genus Coffea L. Seed Sci. Res. 1999, 9, 135–144.
- Dussert, S.; Chabrillance, N.; Engelmann, F.; Anthony, F.; Louarn, J.; Hamon, S. Relationship between seed desiccation sensitivity, seed water content at maturity and climatic characteristics of native environments of nine Coffea L. species. Seed Sci. Res. 2000, 10, 293–300.
- Dickie, J.B.; Pritchard, H.W. Systematic and evolutionary aspects of desiccation tolerance in seeds. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 239–259.
- Ewart, A.J. On the longevity of seeds; Kessinger Publishing: Whitefish, MT, USA, 1908; Volume 21.
- Becquerel, C. La longévité des graines macrobiotiques. C. R. Acad. Sci. Paris 1934, 199, 1662–1664.
- Dum, S. Germination of ancient seeds. Floristical observations and experiments with archaelogically dated soil samples. Dank Bot. Arkiv. 1965, 24, 1–70.
- Posild, A.E.; Harrington, C.R.; Mulligan, G.A. Lupinus arcticus Wats. Grown from seeds of the pleistocene age. Science 1967, 158, 113–114.
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, genetics, and growth of an ancient date seeds. Science 2008, 320, 1464.
- Shen-Miller, J. Sacred lotus, the long-living fruits of China Antique. Seed Sci. Res. 2002, 12, 131–143.
- Lerman, J.C.; Cigliano, E.M. New carbon-14 evidence for six hundred years old Canna compacta seed. Nature 1971, 232, 568–570.
- Côme, D.; Corbineau, F. Dictionnaire de la Biologie des Semences et des Plantules; Lavoisier: Paris, France, 2006; p. 226.
- Bewley, J.D.; Black, M. Physiology and biochemistry of seeds in relation to germination. In Viability, Dormancy and Environmental Control; Spinger: Berlin/Heidelberg, Germany; New York, NY, USA, 1982; Volume 2, p. 375.
- Roberts, E.H. Storage environment and the control of viability. In Viability of Seeds; Roberts, E.H., Ed.; Chapman and Hall: London, UK, 1972; pp. 14–58.
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30.
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409.
- Dickie, J.B.; Ellis, R.H.; Kraak, H.L.; Ryder, K.; Tompsett, P.B. Temperature and seed storage longevity. Ann. Bot. 1990, 55, 147–151.
- Ellis, R.H.; Osei-Bonsu, K.; Roberts, E.H. The influence of genotype, temperature and moisture on seed longevity in chickpea, cowpea and soya bean. Ann. Bot. 1982, 50, 69–82.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. A comparison of low moisture content limit to the logarithmic relation between seed moisture and longevity in 12 species. Ann. Bot. 1989, 63, 601–611.
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Tao, K.L. Low moisture-content limits to relations between seed longevity and moisture. Ann. Bot. 1990, 65, 493–504.
- Walters, C. Deterioration and longevity. In The Encyclopedia of Seeds. Science, Technology and Uses; Black, M., Bewley, J.D., Hamer, P., Eds.; CAB International: Oxfordshire, UK; Cambridge, MA, USA, 2006; pp. 137–138.
- Hay, F.R.; Valdez, R.; Lee, J.-S.; Sta. Cruz, P.C. Seed longevity phenotyping: Recommendations on research methodology. J. Exp. Bot. 2019, 70, 425–434.
- Hay, F.R.; Davies, R.M.; Dickie, J.B.; Merritt, D.J.; Wolkis, D.M. More on seed longevity phenotyping. Seed Sci. Res. 2022, 32, 144–149.
- Newton, R.; Hay, F.; Probert, R. Protocol for comparative seed longevity testing. In Technical Information Sheet 01; Kew, Royal Botanic Gardens: London, UK, 2009.
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. Plant Physiol. 1990, 94, 1019–1023.
- Vertucci, C.W.; Roos, E.E.; Crane, J. Theoretical basis of protocols for seed storage. III. Optimum moisture contents for pea seeds stored at different temperatures. Ann. Bot. 1994, 74, 531–540.
- Chai, J.; Ma, R.; Li, L.; Du, Y. Optimum moisture contents of seeds stored at ambient temperatures. Seed Sci. Res. 1998, 8, 23–28.
- Walters, C.; Engels, J. The effects of storing seeds under extremely dry conditions. Seed Sci. Res. 1998, 8, 3–8.
- Harrington, J.F. Seed storage and longevity. In Seed Biology; Kozlowski, T.T., Ed.; Academic Press: London, UK; New York, NY, USA, 1972; Volume III, pp. 145–245.
- Ellis, R.H.; Hong, T.D. Seed longevity—Moisture content relationships in hermetic and open storage. Seed Sci. Technol. 2007, 35, 423–431.
- Schwember, A.R.; Bradford, K.J. Oxygen interacts with priming, moisture content and temperature to affect the longevity of lettuce and onion seeds. Seed Sci. Res. 2011, 21, 175–185.
- Roberts, E.H.; Abdalla, F.H. The influence of temperature, moisture, and oxygen on period of seed viability in barley, broad beans, and peas. Ann. Bot. 1968, 32, 97–117.
- Bass, L.N. Controlled atmosphere and seed storage. Seed Sci. Technol. 1973, 1, 463–492.
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture, Revised ed.; Food and Agricultural Organization of the United Nations: Rome, Italy, 2014.
- Ballesteros, D.; Pence, V.C. Survival and death of seeds during liquid nitrogen storage: A case study on seeds with short lifespans. CryoLetters 2017, 38, 278–289.
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736.
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916.
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. II. The influence of temperature on optimal moisture levels. Seed Sci. Res. 1993, 3, 201–213.
More
