Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andriani Charpidou and Version 2 by Camila Xu.
Cancer-associated thrombosis (CAT) is a common complication in lung cancer patients. Lung cancer confers an increased risk of thrombosis compared to other solid malignancies across all stages of the disease.
- anticoagulation
- cancer-associated thrombosis
- immune checkpoint inhibitor
- lung cancer
1. Introduction
Venous thromboembolism (VTE) represents a common but challenging disease entity in cancer patients. Approximately 10% of patients with cancer will develop cancer-associated thrombosis (CAT) each year [1]. Cancer represents a hypercoagulable and prothrombotic state, since irregularities may be observed in all elements of the Virchow’s triad: blood flow stasis, endothelial injury, and hypercoagulability [2].
The risk of VTE in cancer patients is 9 times higher than in the general population [3]. CAT is a major cause of death in cancer patients, as the mortality rate of people with cancer with CAT is 2- to 3-fold higher compared with those without CAT [4]. The risk of CAT is dependent on multiple factors, including cancer type, treatment modality, disease stage and time since diagnosis [1], with lung cancer patients being at a high risk of developing CAT. Up to one-fifth of lung cancer (LC) patients may be diagnosed with CAT during the natural history of the disease [5].
Different factors have been implicated in the development of CAT, including abnormalities in platelet count and function, increased expression of prothrombotic genes (particularly tissue factors), the circulation of tumor cells and cancer-associated microparticles, as well as consistent activation of the coagulation pathway [6][7][6,7]. Specifically in LC, increased levels of leukocytes, the generation of neutrophil extracellular traps (NETs), tissue factor-positive (TF+) microvesicles (MVs) and endothelial cell activation have been associated with CAT [8][9][8,9]. Furthermore, it was recently shown that LC patients demonstrate blood hypercoagulability characterized by decreased procoagulant phospholipid-dependent (Procoag-PPL) clotting time, the increased degradation of fibrin and exhausted platelets [9]. Interestingly, the ROADMAP-CAT study provided data suggesting that endothelial cell activation is among the dominant pathophysiological alterations in patients with lung adenocarcinoma [9].
2. Why Do Lung Cancer Patients Have a High Thrombotic Burden?
Multiple factors account for the increased incidence of CAT in LC patients, and can be divided into two categories: individual patient- and cancer-associated factors [2][10][2,10]. Regarding the individual patient-related factors, the impact of age and sex is not clearly defined [2][11][12][13][2,11,12,13]. Co-morbidities such as anemia, obesity and chronic obstructive pulmonary disease (COPD) have been found to increase the VTE risk in LC patients; however, the effects of diabetes, hypertension, pulmonary tuberculosis and cardiovascular disease are not established [12]. Immobilization (can be evaluated clinically by the performance status) is also a significant risk factor, while the effect of smoking appears to be non-significant [2][12][2,12]. Regarding the cancer-related factors, these can be further divided into factors that are associated with the tumor itself or with the anticancer treatment [2][12][13][2,12,13] (Figure 1). LC is usually diagnosed in the advanced or metastatic disease stage [14], which is known to confer an increased risk of thrombosis [15]. The association of locally advanced or metastatic disease and VTE in LC patients has been demonstrated in large-cohort studies (locally advanced stage, adjusted HR: 2.9 (95% CI: 2.3–3.5), p < 0.001; metastatic stage, adjusted HR: 2.5 (95% CI:2.3–2.7), p < 0.001) [16]. VTE occurrence remains an important predictor for early mortality in metastatic NSCLC patients [17].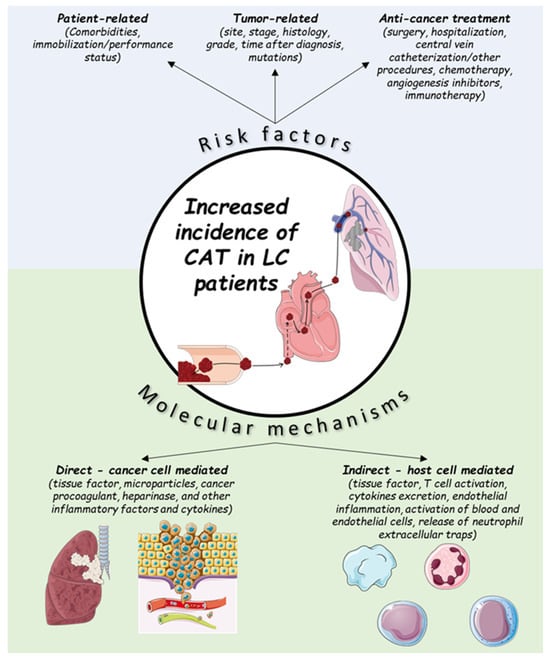
Figure 1.
Risk factors and molecular mechanisms for cancer-associated thrombosis (CAT) in lung cancer (LC) patients.
Table 1.
| Molecular Alteration | CAT Incidence | Effect on CAT Risk |
|---|---|---|
| ALK rearrangement | 26.9–47.1% | 2.2–5 times increase |
| ROS1 rearrangement | 34.6–41.6% | 3–5 times increase |
| KRAS mutation | 16.1–54% | 2.67 times increase |
| EGFR mutation | 9–35% | Conflicting results |
3. Thrombotic Risk Is Related to Antineoplastic Treatment
Cancer patients undergoing systemic treatment for their malignancy are among the highest-risk populations for CAT. The effect of antineoplastic therapy on the overall incidence of CAT is complex, as while systemic treatment may increase the risk of thrombosis, the risk of CAT is reduced when tumor response is achieved [32]. Chemotherapy has been identified as an independent risk factor for CAT events in patients with LC [23][28][33][34][23,28,33,34]. The real-life incidence of CAT in patients with LC receiving chemotherapy has been reported as 14.1% at six months after the start of chemotherapy [35]. The risk of CAT is not consistent among all cytotoxic agents, including agents with the same mechanism of action [32]. In particular, platinum-based chemotherapy, which is widely used in LC patients, may increase the incidence of CAT. A retrospective analysis by the Memorial Sloan Kettering Cancer Center found that 18.1% of cancer patients developed thrombosis during cisplatin treatment within 4 weeks after chemotherapy [34]. Different mechanisms have been implicated in the development of chemotherapy-induced thrombosis, including direct endothelial damage and increased tissue factor activity [36]. The exact mechanisms of thrombosis for carboplatin and cisplatin, which are widely used in the treatment of LC, have yet to be determined [37]. LC patients with driver mutations receiving targeted therapies are also at a high risk of CAT [38]. However, as some driver mutations in LC have also been associated with an increase in risk (ALK, ROS1), it has not been established if the use of targeted agents has a beneficial or deleterious effect on the risk of CAT [29]. A recent retrospective cohort study suggested that the use of TKIs does not increase the risk of thrombosis, as the initiation of targeted therapy is not associated with an increased risk of CAT [39]. The mechanism of targeted therapy-induced thrombosis may be different depending on the type of agent used. The use of anti-vascular endothelial growth factor (VEGF) agents in advanced NSCLC has been associated with an increased risk of high-grade arterial thromboembolism, but not VTE [40]. The risk of CAT in patients receiving a combination treatment with targeted agents may be increased [41]. CAT represents a major contributor to morbidity and mortality in patients with early and locally advanced LC receiving treatment with curative intent [42]. Even in early stages, LC patients are in a hypercoagulable state characterized by the increased generation of thrombin and phosphatidylserine expressing platelet-derived microparticles (Pd-MP/PS+). This hypercoagulable state is not sufficiently corrected after lung lobectomy [43]. Locally advanced disease, requiring open and more extensive resections, has been associated with an increased risk of CAT, which doubles the risk of 1-year mortality in this population [42]. The use of radiotherapy has also been shown to increase the risk of CAT in LC patients [22]. Supportive treatments, including the use of red blood cell (RBC) transfusions, erythropoietin stimulating agents (ESAs) and central venous catheters (CVC), have been associated with CAT [44]. However, in a retrospective analysis of CAT trends in chemotherapy-induced anemia, the use of RBC transfusions or ESAs did not alter the risk of thrombosis in LC patients [45]. In contrast, CVC-related thrombosis (CRT) is a common complication in patients with indwelling central venous access devices. The widespread use of CVC in LC patients has altered the clinical presentation of CAT, including by increasing the rate of DVT in the upper extremities.Immune Checkpoint Blockade
The introduction of immune checkpoint inhibitors has altered the treatment landscape of LC, with demonstrably large benefits in overall survival but largely unknown effects on the risk of CAT. CAT is common in cancer patients receiving immunotherapy either as single-agent or combination regimens [46]. Lung cancer patients receiving immune checkpoint inhibitors may demonstrate a similar or higher risk of CAT compared to patients receiving chemotherapy, with the highest risk group being patients receiving combinations of chemotherapy and immunotherapy [38][47][38,47]. The real-life incidence of CAT in LC patients receiving therapy with immune checkpoint inhibitors is higher than 10% [48]. The use of combination immunotherapy with PD-1 and CTLA-4 blockade may increase the risk of CAT over PD-1 blockade alone (1-year incidence: 29.3% vs. 9.1–14.9%, p < 0.05) [49]. Pulmonary embolism is the cause of a significant proportion of CAT events in patients receiving immunotherapy [48]. CAT may be associated with higher mortality in this population, although this effect has not been consistently demonstrated across all studies [50]. The interplay between thrombosis and immune response, also described as immunothrombosis, has been well-established, most recently in patients with uncontrolled immune activation caused by COVID-19 [51]. In cancer patients, the formation of altered cell components during carcinogenesis that trigger the activation of innate immunity may promote immune-mediate thrombosis [52]. This process has been suggested to promote immune evasion and to inhibit the response to immune checkpoint blockade in cancer patients by altering T cell responses [53]. Furthermore, the development of fibrin clots alters the tumor immune microenvironment and may further inhibit immune response by presenting a physical barrier for infiltrating immune cells [54]. On the other hand, sustained inflammation has been associated with CAT and tumor metastasis in preclinical lung cancer models, including by the formation of neutrophil extracellular traps [55], which have been implicated in venous and arterial thrombosis as a response to inflammation [56]. Other suggested mechanisms of immunothrombosis in LC patients include the formation of platelet-T cell aggregates and the increased expression of tissue factors in circulating monocytes [20][57][20,57]. The exact mechanism for the development of CAT in LC patients receiving checkpoint immunotherapy has not been established. Factors supporting the inflammatory theory of thrombosis in patients receiving immune checkpoint inhibitors include the increased risk of thrombosis in patients receiving combination immunotherapy or experiencing immune-related adverse events, as well as correlations between CAT and inflammatory biomarkers such as C-reactive protein, interleukin-8 and myeloid-derived suppressor cells [49][58][59][49,58,59]. The PD-1/PD-L1 axis may also be directly involved, as PD-L1 positivity has been associated with an increased risk of VTE [60]. The potential mechanisms of immunothrombosis in LC are summarized in Table 2.Table 2.
Proposed mechanisms of immunothrombosis in LC patients.
| Interaction of Thrombosis with Immune Response | Interaction of Immune Response with Thrombosis |
|---|---|
| Immune invasion | Neutrophil extracellular traps |
| Diminished T-cell response | Platelet T-cell aggregates |
| Altered tumor immune microenvironment | Tissue factor-positive monocytes |
4. Risk Assessment Models (RAMs) for Cancer-Associated Thrombosis
The first RAM for CAT in outpatients with solid tumors, the Khorana Risk Score (KRS), was presented by Khorana et al. in 2008 [65]. The predictors of the KRS include the tumor type dichotomized to very high-risk cancers (stomach, pancreas) and high-risk cancers (lung, lymphoma, gynecologic, bladder, testicular, renal). The KRS also includes the body mass index as a patient-related factor. Lastly, the KRS includes hematological markers (pre-chemotherapy levels of hemoglobin, platelets and white blood cell count) that are non-specific for blood hypercoagulability. The original KRS has been derived and validated to evaluate VTE risk in outpatients before the initiation of chemotherapy [66]. The KRS may also be useful in hospitalized cancer patients. In a study by Patell et al., patients with a high KRS (≥3) were significantly more likely to develop VTE during hospitalization than patients with a low KRS (multivariable OR, 2.52; 95% CI, 1.31 to 4.86). Similar results were reported in a multicenter retrospective study of 1398 hospitalized patients [67][68][67,68]. In this analysis, in-hospital VTE occurred in 5.4% (95% CI, 1.9% to 8.9%) of high-risk patients, 3.2% (95% CI, 2.0% to 4.4%) of intermediate-risk patients, and 1.4% (95% CI, 0.3% to 2.6%) of low-risk patients (OR for high- low-risk patients, 3.9; 95% CI, 1.4 to 11.2). However, the KRS has several limitations as a predictive and prognostic tool in clinical practice. In a recent multicenter prospective observational study in Japan, the KRS was evaluated in 1008 patients with advanced lung cancer, of whom 10% developed CAT. VTE risk could not be determined because both the Khorana score and modified Khorana score, based on BMI targets adjusted for the Asian population, showed very low areas under the curve (0.518 and 0.516, respectively) [69]. This result was replicated in a large systematic review and meta-analysis of 55 cohorts, where the KRS was of limited use for predicting future risk of CAT in LC patients [66]. Furthermore, the CASSINI study, a double-blind, randomized trial involving 1080 high-risk ambulatory patients with cancer (KRS of ≥2, on a scale from 0 to 6) who received either rivaroxaban or placebo for up to 6 months, did not demonstrate any significant decrease in 6-month symptomatic VTE rate in the treatment group (6%) versus the control group (8.8%; hazard ratio, 0.66; 95% confidence interval (CI), 0.40 to 1.09; p = 0.10) [70]. In contrast, AVERT, a double-blind trial of apixaban designed similarly to CASSINI, showed a significantly reduced 6-month rate of VTE (4.2%) in the apixaban group as compared to the placebo group (10.2%; hazard ratio, 0.41; 95%; CI = 0.26 to 0.65; p < 0.001) [71]. The aforementioned limitations of the KRS have led to the development of other RAMs, derived from prospective observational studies, although none of them have been externally validated in cancer patients as extensively as KRS. The clinical, biochemical and genetic criteria used for the assessment of thrombotic risk in the different RAMs are summarized in Table 3.Table 3. Risk assessment models for cancer-associated thrombosis. Score criteria are provided where appropriate. Abbreviations: BC—breast cancer; CAT—cancer-associated thrombosis; ChT—chemotherapy; Hb—hemoglobin; KRS—Khorana risk score; VTE—venous thromboembolism; WBC—white blood cell. Cardiovascular risk is defined as at least 2 of the following: peripheral artery disease, ischemic stroke, coronary artery disease, hypertension, hyperlipidemia, diabetes, obesity.
| Criteria | KRS | Vienna-CATS | CONKO | ONCOTEV | PROTECHT | COMPASS-CAT |
|---|---|---|---|---|---|---|
| Very high-risk tumor (stomach, pancreas) |
[93,94]. Abbreviations: ESA, erythropoiesis-stimulating agent; G-CSF, granulocyte colony-stimulating factor; P-gp, P-glycoprotein.
| Category | Agent | CYP3A4 Interactions | P-gp Interactions | |||||
|---|---|---|---|---|---|---|---|---|
| 2 | 2 | 2 | 2 | |||||
| Corticosteroids | Dexamethasone | Strong inducer and substrate | No | |||||
| High-risk tumor (lung, lymphoma, gynecologic, bladder, testicular) | 1 | 1 | 1 | 1 | ||||
| Prednisolone | Moderate inducer and substrate | Inhibitor and Substrate | Pre-ChT platelet count ≥ 350 × 109/L | 1 | 1 | 1 | ||
| Bisphosphonates and Denosumab | Zoledronic acid | 1 | 2 | |||||
| No | No | Hb ≤ 100 g/L or use of red cell growth factors | 1 | 1 | 1 | 1 | ||
| Denosumab | No | No | Pre-ChT WBC ≤ 11 × 109/L | 1 | 1 | |||
| Antiemetics | Ondansetron | 1 | 1 | |||||
| Substrate | Substrate | BMI ≥ 35 kg/m2 or more | 1 | 1 | 1 | |||
| Palonosetron | Substrate | No | 1 | |||||
| D-dimer > 1.44 μg/L | 1 | |||||||
| Metoclopramide | No | No | Soluble P-Selectin > 53.1 ng/L | 1 | ||||
| Aprepitant | ||||||||
| Moderate inhibitor and substrate | WHO performance status ≥ 2 | 1 | ||||||
| No | Gemcitabine ChT | 1 | ||||||
| Platinum-based ChT | 1 | |||||||
| KRS > 2 | 1 | |||||||
| Previous VTE | 1 | 1 | ||||||
| Metastatic disease | 1 | |||||||
| Vascular/lymphatic macroscopic compression | 1 | |||||||
| Anti-hormonal therapy for BC or anthracycline ChT | 6 | |||||||
| Time since cancer diagnosis ≤6 months |
4 | |||||||
| Central venous catheter | ||||||||
| Fosaprepitant | Moderate inhibitor and substrate | No | ||||||
| Analgesics and anxiolytics | Oxycodone | Substrate | No | |||||
| Hydromorphone | No | No | ||||||
| Morphine | No | No | ||||||
| Fentanyl | Weak inhibitor and substrate | No | ||||||
| Paracetamol | Weak inhibitor and substrate | No | ||||||
| Lorazepam | No | No | ||||||
| Clonazepam | Substrate | No | ||||||
| G-CSF | Filgrastim | 3 | ||||||
| Advanced disease | 2 | |||||||
| Cardiovascular risk | 5 | |||||||
| Recent hospitalization for acute medical illness | 5 | |||||||
| Low | 0 | 0 | 0 | 0 | 0 | 0–6 | ||
| Intermediate | 1–2 | 1–2 | 1–2 | 1 | 1–2 | |||
| High | ≥3 | ≥3 | ≥3 | ≥2 | ≥3 | ≥7 |
5. Evaluation of the Bleeding Risk
Bleeding is a significant challenge for patients with advanced solid tumors, with approximately 10% of all cancer patients having at least one episode [72]. Hemoptysis is among the most common respiratory symptoms in LC, and approximately 20–60% of patients with LC will experience some degree of hemoptysis during the natural history of the disease [73][74][75][73,74,75]. In total, 5–10% of episodes of hemoptysis are considered severe (blood loss > 100 mL/day), and without timely management, the mortality rate exceeds 50%. Chemotherapy-induced thrombocytopenia (CIT) (defined as platelet count < 100 × 109/L) can delay antineoplastic treatments or surgical procedures, while increasing the likelihood of serious bleeding events eventually resulting in hospitalization [76]. Importantly, while thrombopenia increases the risk of bleeding, it does not reduce the risk of CAT [77], complicating the use of anticoagulation, as most RCTs that evaluate its benefits in CAT exclude this patient population [78][79][80][81][78,79,80,81]. In addition, LC patients may develop organ insufficiencies either as a result of their malignancy or from adverse effects of different treatment modalities, including chemotherapy, immunotherapy and targeted therapy. In particular, renal and hepatic insufficiency increase the risk of both CAT and bleeding, while simultaneously affecting the pharmacokinetics of different anticoagulation agents [82][83][84][82,83,84]. Particularly for LC, squamous histology, vascular invasion, central location, and history of hemoptysis are related to an increased risk of bleeding. A careful and comprehensive evaluation of the patient with active LC and an individualized approach tailored to the patient’s bleeding risk are key to the assessment and management of anticoagulation for CAT. In particular, clinicians should assess:-
The particular characteristics of LC in the evaluated patient, including lung cancer histology, the stage and resectability of the disease, the invasion of large vessels, the presence of active brain metastases, the response to antineoplastic treatment and the presence of cancer cachexia;
-
The medications of the patient, including present antineoplastic and anticoagulation therapy and possible drug–drug interactions;
-
The personal history of bleeding or thrombosis;
-
The existence of comorbidities that exacerbate the risk of bleeding or CAT, including thrombopenia, renal or hepatic insufficiency, gastrointestinal and other disorders, as well as the expected duration and potential reversibility;
-
The prognosis of the disease, the intent of treatment (curative or palliative) and patient preferences
6. Considerations when Choosing the Optimal Anticoagulant
At this point in time, the benefit of anticoagulation in CAT is well established. Large meta-analyses of RCTs of ambulatory patients that included LC patients have shown that both LMWHs and direct-acting oral anticoagulants (DOACs) confer an approximate 50% reduction in the risk of CAT without increasing the risk of major bleeding [86][87][86,87], and therefore are reasonable options. In LC patients at higher risk of bleeding (squamous histology, vascular invasion, central location, history of hemoptysis), it is reasonable to prefer LMWHs over DOACs due to the potentially lower risk of bleeding, based on indirect evidence [78]. Regardless of the specific agent chosen, patients at high risk of CAT should receive prophylactic anticoagulation for at least 6 months, with treatment beyond 6 months being a reasonable option for individual patients at very high risk of CAT [88]. In patients hospitalized for an acute medical illness, LMWHs should be preferred, as DOACs are associated with an increased risk of bleeding in this setting [88]. Vitamin K antagonists are less effective in reducing the risk of CAT and should be considered a second-line option for prophylaxis in patients ineligible for treatment with LMWHs or DOACs [89]. As the benefits and risks of different anticoagulation agents are similar in many cases, the selection of the optimal treatment strategy for the management of CAT should be individualized, based on the risk of thrombosis and bleeding, the potential for drug–drug interactions (DDIs), polypharmacy and patient preferences. Polypharmacy, defined as the use of five or more medications, is common in patients with cancer, who are often treated with multiple antineoplastic and supportive therapies. The risk of major DDIs in cancer patients increases linearly from 14% in patients receiving less than four medications to 67% in patients receiving more than 11 medications [90]. Furthermore, the use of two or more medications has been associated with an increased risk of major bleeding in patients receiving anticoagulation for VTE or atrial fibrillation [91]. It is thus important to evaluate the potential for drug–drug interactions when selecting the appropriate anticoagulation therapy for CAT. All DOACs are substrates of P-glycoprotein and cytochrome P450, so therapies that affect P-glycoprotein or CYP3A4 metabolism have the potential to interact with DOACs [92]. Numerous anticancer therapies are inhibitors or inducers of the P-glycoprotein and/or CYP3A4 pathways, with the potential to interact with DOACs [93]. LC therapies with the potential for DDIs with DOACs, along with their bleeding, gastrointestinal and hematological implications. Despite fewer interactions with DOACs, physicians need to also consider the pharmacokinetic DDIs of supportive care drugs and comorbidities when prescribing DOACs. On the other hand, there is little risk of pharmacokinetic DDIs with LMWHs [83]. The potential DDIs of supportive oncology care drugs are depicted in Table 4.| No |
| No |
| ESA |
| Epoetin alfa/beta |
| No |
| No |
| Darbepoetin alfa |
| No |
| No |
